Mass Spectrometry
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
How can the average mass of an atom be calculated?
Using the mass of the isotopes of an element along with their relative abundances.
What is a mass spectrometer?
A mass spectrometer is a scientific instrument that is used to analyze the mass and structure of molecules and atoms.
Summarise the processes that take place in a mass spectrometer.
Vapourisation
Ionisation
Acceleration
Deflection
Detection
Describe the process of vapourisation that occurs in a mass spectrometer.
The sample (containing all the different isotopes of the element) is heated and turned into a gas before it enters the spectrometer.
Describe the process of ionisation that occurs in a mass spectrometer.
The gaseous sample is bombarded with high energy electrons. This causes electrons to be removed from the atoms (it is ionised), leaving +1 positive ions in the chamber.
Describe the process of acceleration that occurs in a mass spectrometer.
The positively charged ions are attracted to the negatively charged plates in an electric field, causing them to accelerate.
Describe the process of deflection that occurs in a mass spectrometer.
The ions are deflected by a magnetic field. The amount of deflection depends on the mass and charge of the ion (lighter ions are deflected more than the heavier ones).
Describe the process of detection that occurs in a mass spectrometer.
Some ions pass through a slit and are detected by the ion detector. Only those ions of the correct mass/charge ratio will be able to pass through the slit.
Why is the inside of the mass spectrometer under vacuum conditions?
Having a vacuum reduces the chance of ions colliding with other molecules which could interfere with the mass spectrum produced.
What is a mass spectrum?
The resulting record of the ion peaks.
The abudance of all of the isotopes of an element added together is…
100.
How can the relative atomic mass of an element be determined from a mass spectrum?
(Relative isotopic mass of isotope 1 x Abudance) + (Relative isotopic mass of isotope 2 x Abudance) / 100.
What type of molecule is chlorine?
Diatomic– it exists as two atoms of chlorine covalently bonded together.
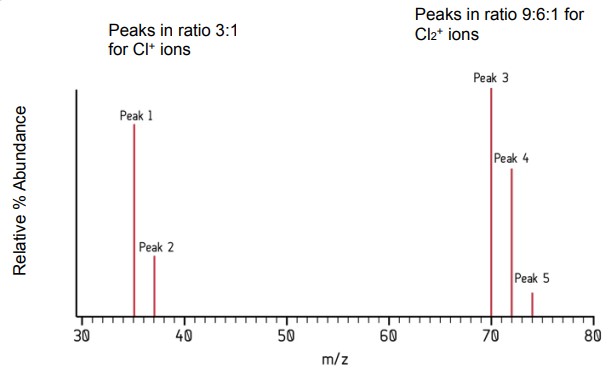
Explain the peaks produced on the mass spectrum of chlorine.
As chlorine is a diatomic molecule, the mass spectrum of chlorine therefore includes peaks for chlorine atoms (which exists as two isotopes) and chlorine molecules (which can exist in any combination of the two isotopes).
What 2 things can occur when chlorine molecules enter the ionisation chamber of a mass spectrometer?
They can have one electron knocked off the molecule to give a molecular ion, Cl2+.
The unstable molecular ion will fall apart to give a chlorine atom and a chlorine ion, Cl+. (Fragmentation) The atom formed here is also ionised.
Analyse the mass spectrum of chlorine.
Peak 1 is caused by 35Cl and Peak 2 by 37Cl. These are in the ratio of 3:1, which means that the 35Cl isotope is three times more likely to exist than the 37Cl isotope.
In order to explain the 9:6:1 ratio for the molecular ions, we have to think about all the possible combinations of Cl2 molecules that can be made from the two isotopes:
(35Cl -35Cl)+ - this is responsible for Peak 3 at m/z = 70
(35Cl – 37Cl)+ and (37Cl – 35Cl)+ - these are responsible for Peak 4 at m/z = 72
(37Cl – 37Cl)+ - this is responsible for Peak 5 at m/z = 74