Chemistry - Chapter 24: Electrochemistry
1/10
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Electrode potential
The voltage measure for a half-cell compared with another cell. Shows how easily a substance can be reduced
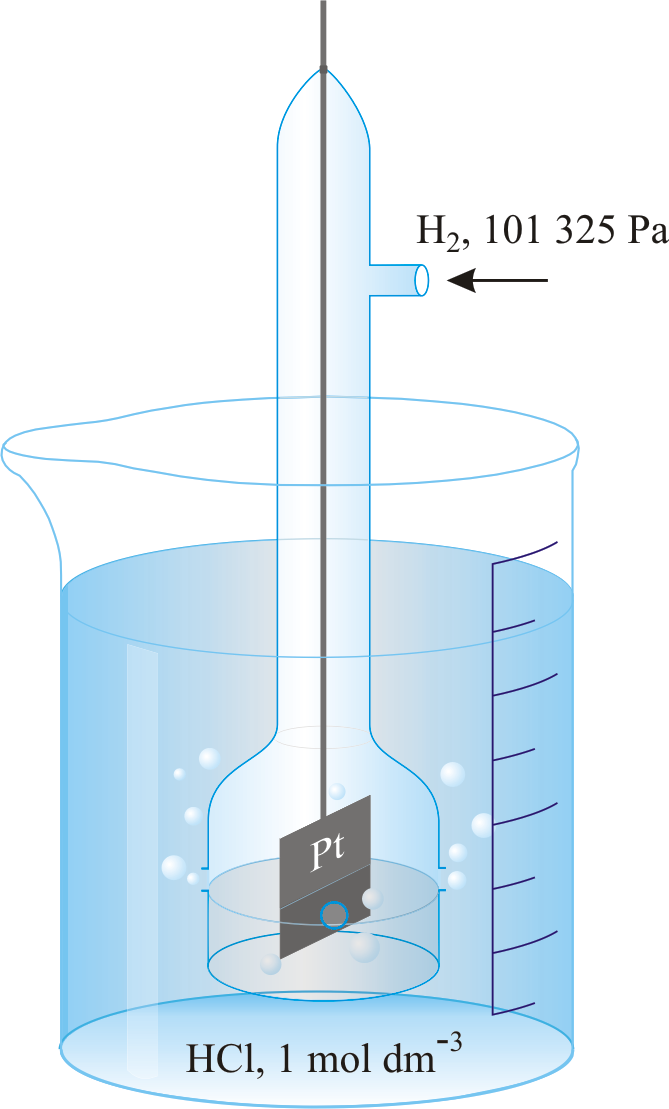
Standard hydrogen electrode
A half-cell in which H2 gas at pressure 101 kPa bubbles through a solution of 1 mol.dm-3 of H+ ions
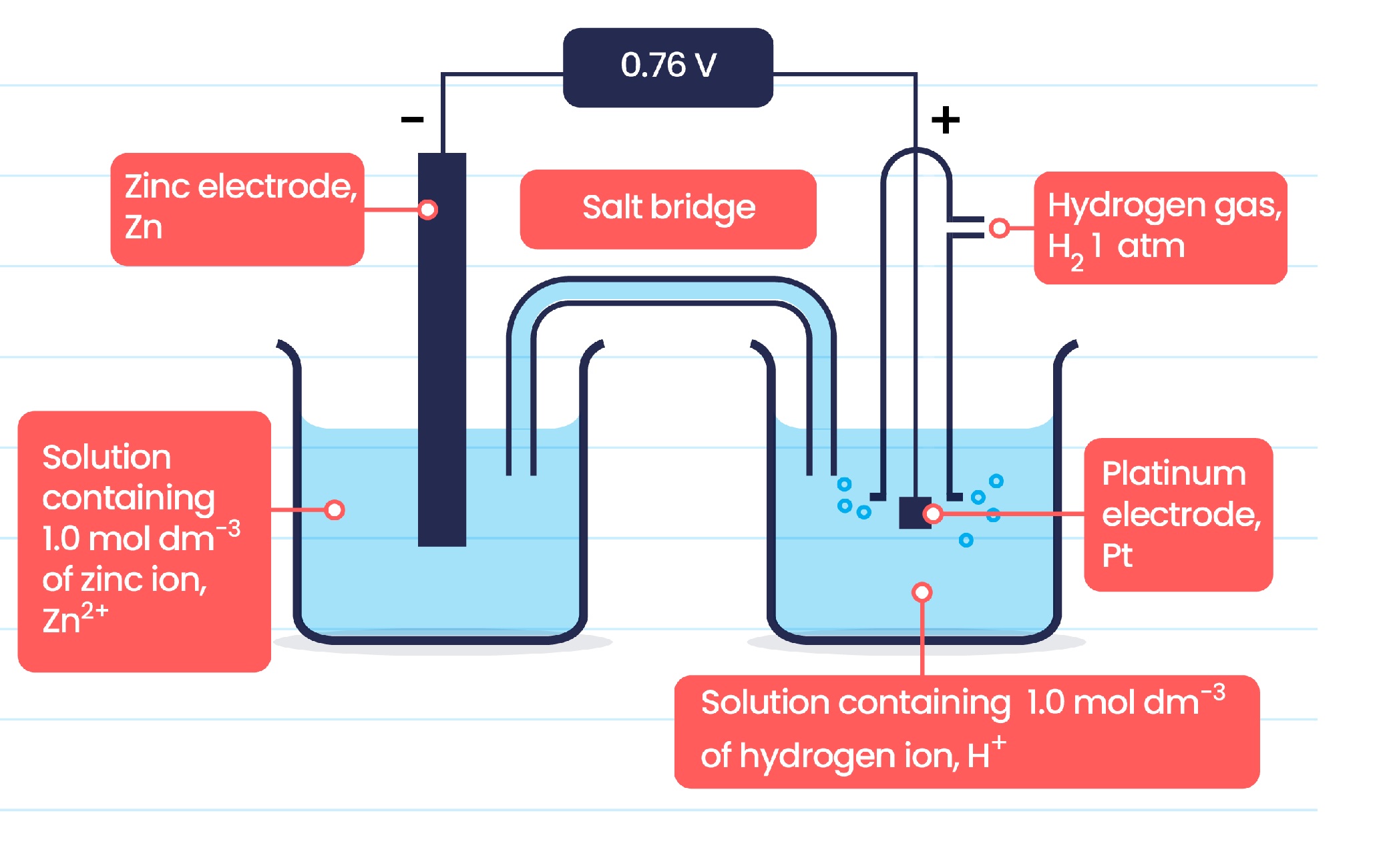
Standard electrode potential
The voltage produced when a standard half-cell is connected to a standard hydrogen electrode under SD
Standard cell potential
The difference in standard electrode potential between 2 specific half-cells
Half-cell
One half of an electrochemical cell which either donates or receives electrons from an external circuit when connected to another half-cell
Electrolysis
The decomposition of an ionic compound when it is a molten/aqueous solution by an electric current
Electrolyte
A molten ionic compound or an aqueous solution of ions that is decomposed during electrolysis
Electrodes
A rod of metal or carbon (graphite) which conducts electricity to or from an electrolyte
Anode
The positive electrode
Cathode
The negative electrode
Faraday
The quantity of electric charge (in coulombs) carried by one mole of electrons or one mole of singly charged ions