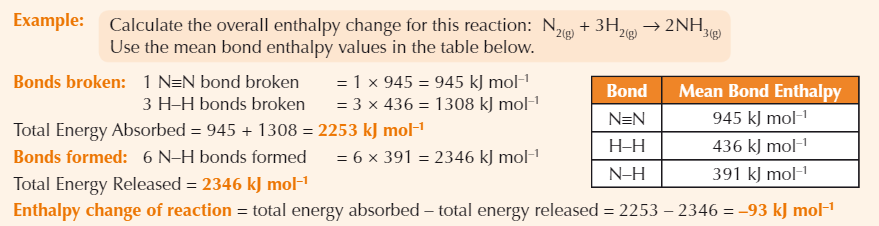3.1.4.4 - Bond enthalpies
1/6
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Bond breaking
Endothermic - requires energy to break bonds
ΔH positive
Stronger bonds take more energy to break
Bond making
Exothermic - releases energy by breaking bonds
ΔH negative
Stronger bonds release more energy when they form
Bond enthalpy
The energy required to break bonds
Mean bond enthalpy
Average energy needed to break a certain type of bond, over a range of compounds
Always positive - breaking bonds = endothermic

Bond enthalpy variation
Energy needed to break a bond depends on its environment → same type of bond may require different amounts of energy to break
Enthalpy change of reaction formula
Enthalpy change of reaction = total energy absorbed - total energy released
Example bond enthalpy calculation