ACIDOS Y BASES | Quizlet
0.0(0)
Card Sorting
1/11
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
1
New cards
pH=
-log[H+]
2
New cards
pOH=
-log[OH-]
3
New cards
pH+ pOH=
14
4
New cards
[H+]=
10^-pH
5
New cards
[OH-]=
10^-pOH
6
New cards
Kw(producto ionico del agua)=
[H+][OH-] = 10^-14
7
New cards
Ka=
[ ]^2/[acido/base]
8
New cards
tipos de soluciones amortiguadora
sistema acido-sal, base-sal y salino
9
New cards
que es una solucion amortiguadora?
aquella que resiste a los cambios bruscos de pH
10
New cards
Ecuación de Henderson- Hasselbach
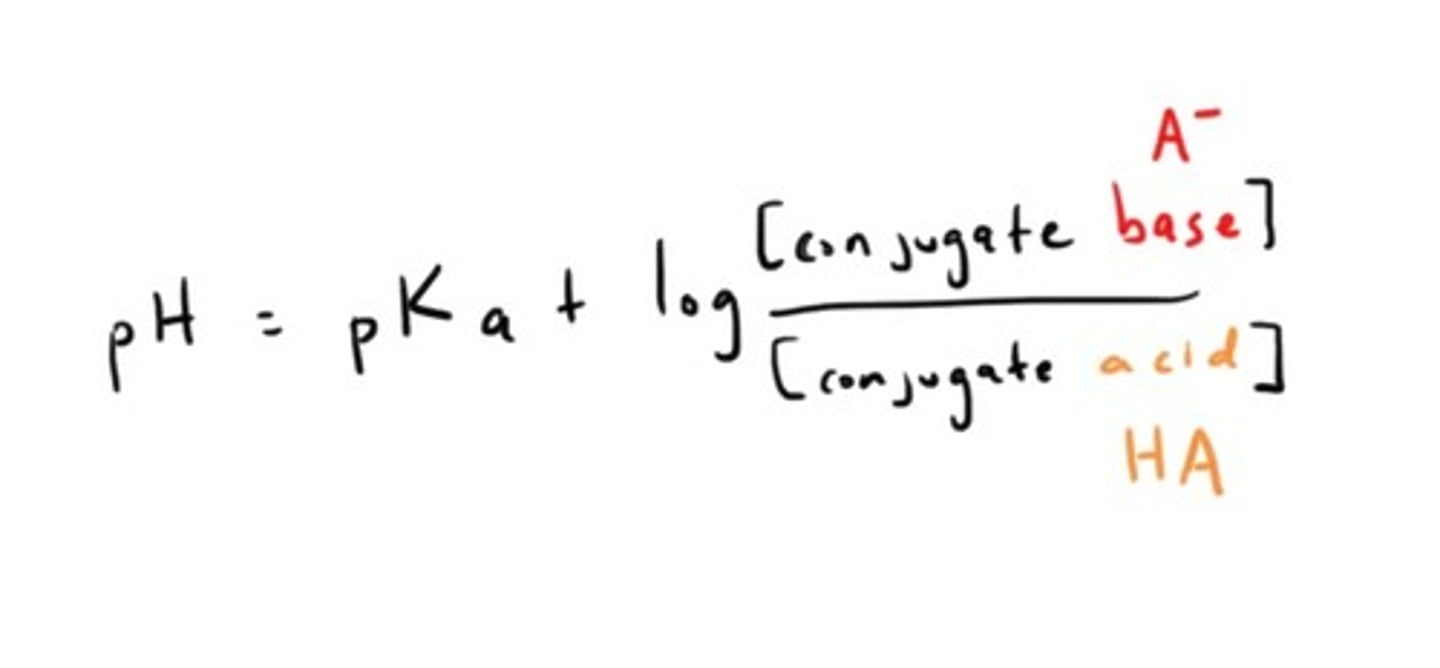
11
New cards
el pKa y Ka para que sirven??
para medir si hay acidos debiles o fuertes. El Ka es a escala pequeña y pKa a grande asi como el pH
12
New cards
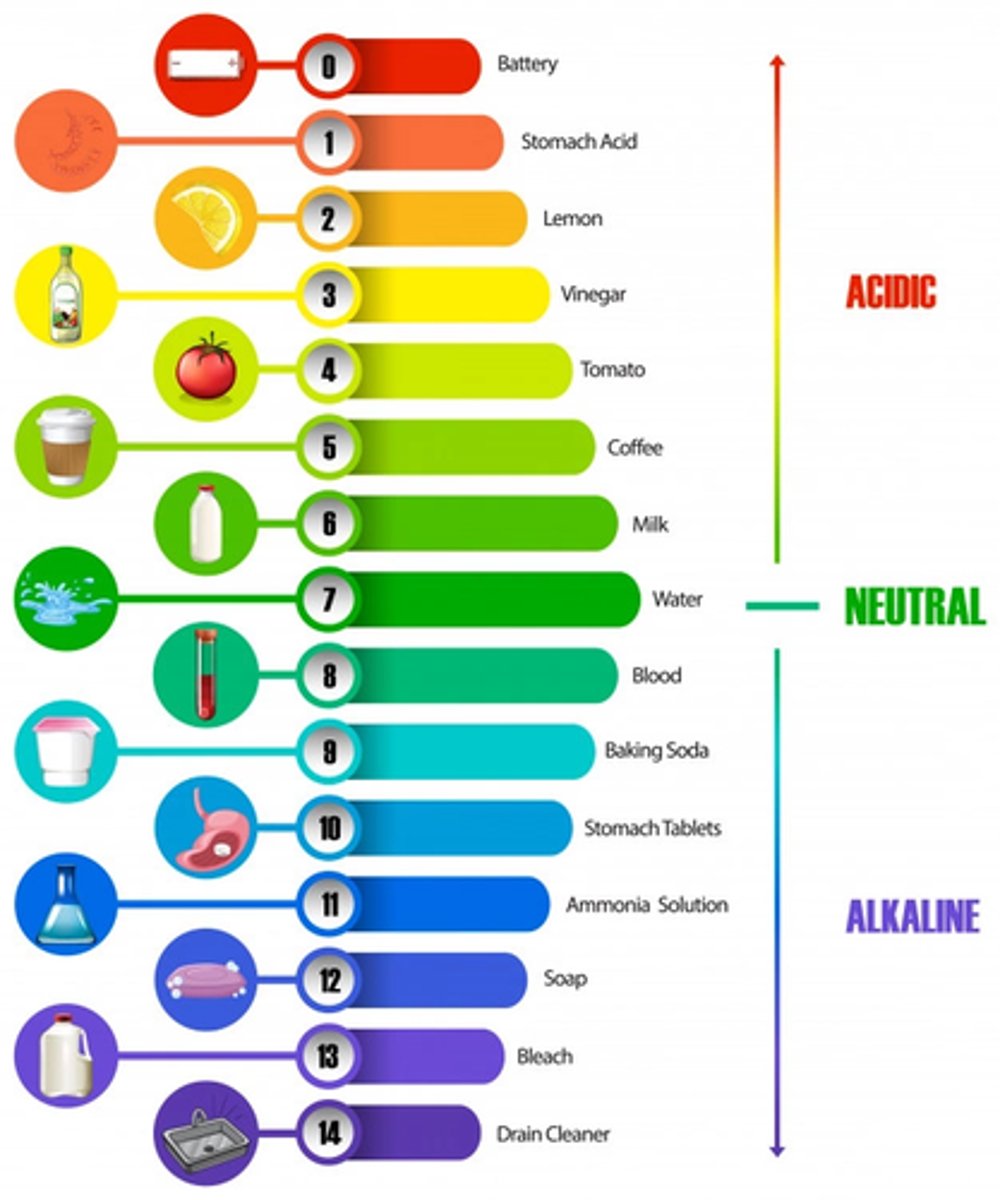
Señalar la escala de pH
acido < 7 y base > 14