Need to Know
1/119
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
120 Terms
Nitrogen (Group V-VIII Fun Facts)
Can assume many oxidation states (-3 to +5).
It is found in explosives, fertilizers (ammonia is made with the Haber process from H2 and N2 at low temperature with a catalyst),
and as an oxide in laughing gas, Viagra, smog, and preservatives.
Phosphorous (Group V-VIII Fun Facts)
found in man common materials including soaps, toothpaste, fertilizer, and pesticides.
It is extracted from Ca3(PO4)2 rock.
Phosphate is the backbone in nucleic acids like DNA.
Phosphates cause algae bloom and increasingly their use is discouraged for environmental reasons.
H2SO4 (Main Group V-VIII Fun Facts)
the chemical manufactured in the largest quantities; it is made by a two-step process, Claus and contact, that converts H2S extracted from petroleum into H2SO4.
It has many uses as a strong acid, oxidizing agent, and dehydrating agent, including extraction of phosphate from rock, production of paper, and as a reactant in production of many other important chemicals.
Halides (Main Group V-VIII Fun Facts)
have small radii, high ionization energy, high electronegativity, and form -1 anions.
Their oxides and hydrides are acidic.
Fluoride (F–) (Main Group V-VIII Fun Facts)
inserts instead of OH– in tooth enamel to protect from decay
Chlorine (Main Group V-VIII Fun Facts)
is manufactured as Cl2, a strong oxidizing agent.
It is used in disinfection and sanitation.
It is also used to make PVC tubing.
Noble gases (Main Group V-VIII Fun Facts)
are inert with 2 or 8 electrons in filled shells.
Specialty uses include as cryogens (He), inert gases (Ar), and lights (Ne).
Wet method (Reactants)
Ca10(PO4)6F2, H2SO4
Wet method (Products)
H3PO4
Claus (Reactants)
H2S
Claus (Products)
S
Contact (Reactants)
S
Contact (Products)
H2SO4
Claus and Contact processes
combine to produce H2SO4 through a series of oxidation and acid/base reactions.
Claus
A two-step oxidation produces elemental sulfur from H2S, which is a contaminant in natural gas, methane.
Contact
A four-step oxidation process produces H2SO4 from elemental sulfur, S.
Fun sulfuric acid facts
• It is the most manufactured chemical in the world.
• About half of all sulfuric acid is used to solubilize
phosphate in rocks by the wet method. In turn the
solubilized phosphate is used in fertilizers.
• Sulfuric acid is not just a strong acid. It is also a strong
oxidizing agent and a strong dehydration agent (removing
water).
Redox reactions are the
basis for electrochemistry
Free elements have an oxidation # of
0
Individual ions are
assigned oxidation numbers based on their charge, reflecting the number of electrons lost or gained.
alkali metals (Group I) have
an oxidation number of +1
Hydrogen has
an oxidation number of +1 in most compounds
H has -1 charge in
NaH
Oxygen has
an oxidation number of -2
O has -0.5 charge in
NaO2
O has -1 charge in
H2O2
O has -2 charge in
MgO
oxidation numbers are typically assigned by
group on periodic table
Group I Oxidation Number
+1
Group II Oxidation Number
+2
Group III Oxidation Number
+3 or -5
Group IV Oxidation Number
+4 or -4
Group V Oxidation Number
-3 or +5
Group VI Oxidation Number
-2
Group VII Oxidation Number
-1
Sum of individual charges must
equal overall charge on molecule
OIL RIG
• Oxidation is Loss of electrons
• Reduction is Gain of electrons
oxidizing agent
is the species that causes oxidation of other species.
This is the species that is being reduced.
reducing agent
is the species that causes reduction of other species.
This is the species that is being oxidized.
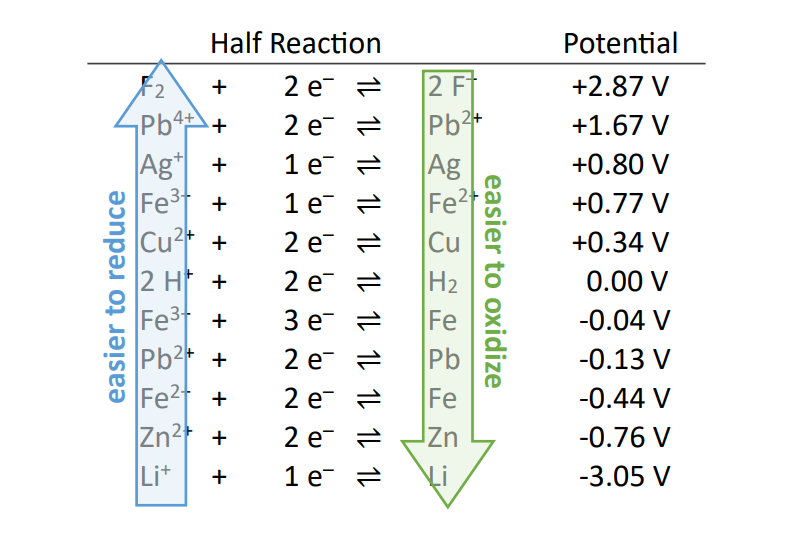
As you move UP the table, the species on the left are
EASIER TO REDUCE (stronger oxidizing agents)
Part of the half-cell with the largest POSITIVE reduction potential.
As you move DOWN the table, the species on the right are
EASIER TO OXIDIZE (stronger reducing agents)
Part of the half-cell with the largest NEGATIVE reduction potential.
Table of Reduction Potentials
Steps for Balancing in Neutral Water
1. Assign oxidation numbers
2. Create brackets for oxidation and reduction
3. Find least common multiple and balance. This is n.
Steps for Balancing in ACID
1. Assign oxidation numbers
2. Create brackets for oxidation and reduction
3. Find least common multiple and balance. This is n.
4. In acid, find deficient O and put the same # of H2O on
deficient side and double H+ on opposite side.
Steps for Balancing in BASE
1. Assign oxidation numbers
2. Create brackets for oxidation and reduction
3. Find least common multiple and balance. This is n.
4. In base, find deficient O and put same # of H2O on
opposite side and double OH– on the deficient side.
Battery (Voltaic, Galvanic) Delta G
-
Battery (Voltaic, Galvanic) K
> 1
Battery (Voltaic, Galvanic) E
+
Battery (Voltaic, Galvanic) Reduction
At Cathode
Battery (Voltaic, Galvanic) Oxdation
At Anode
Battery (Voltaic, Galvanic) Electron Flow
Anode to Cathode
Battery (Voltaic, Galvanic) Cathode
+
Battery (Voltaic, Galvanic) Anode
-
Electrolytic Delta G
+
Electrolytic K
< 1
Electrolytic E
-
Electrolytic Reduction
At Cathode
Electrolytic Oxidation
At Anode
Electrolytic Electron Flow
Anode to Cathode
Electrolytic Cathode
-
Electrolytic Anode
+
Given shorthand cell notation, you should know/be able to explain
• Cathode and anode
• What happens chemically
• What is produced/consumed
• In plain language, explain what is happening
to the electrodes
• Cell conventions (signs, e– flow)
• The role of Pt or Au electrodes
Zn | Zn2+ || Cu2+ | Cu
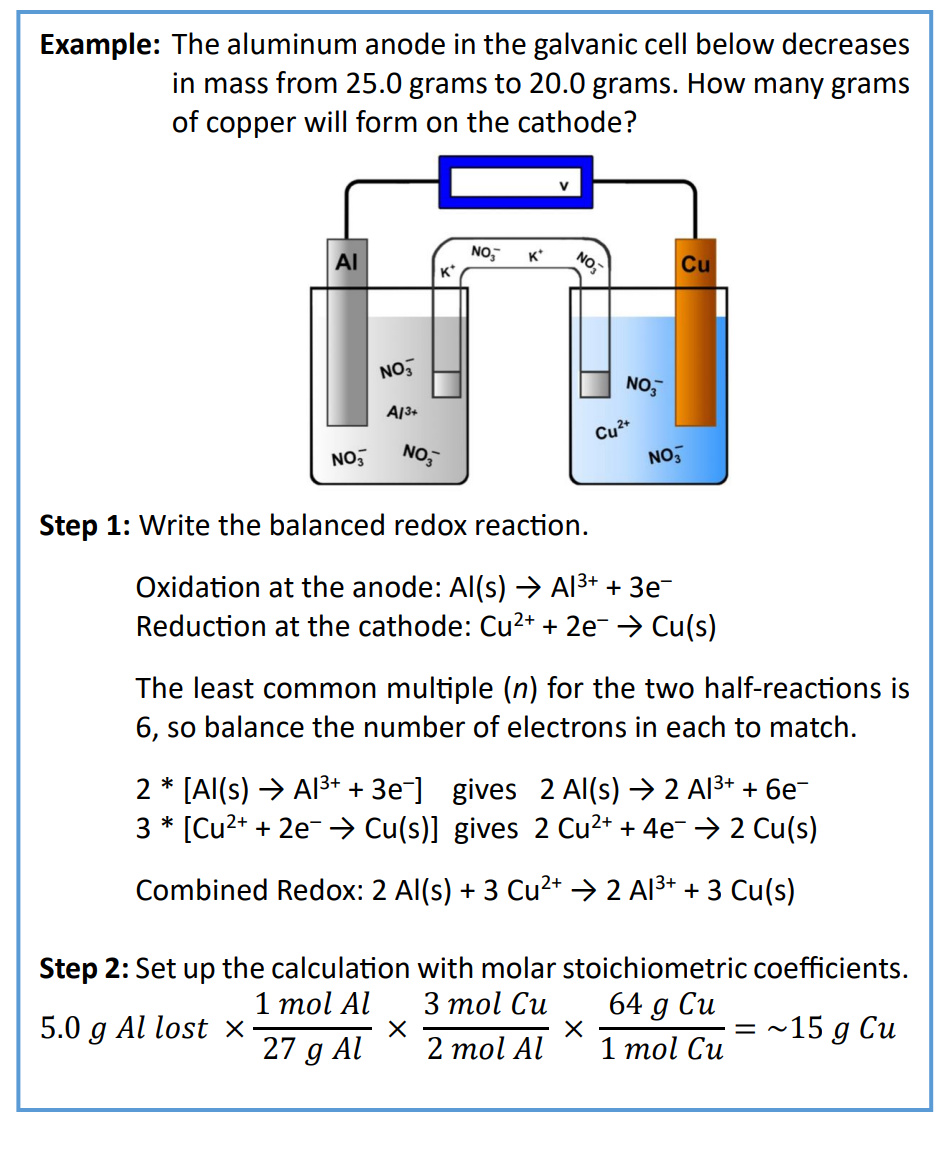
The very left and very right sides are the electrodes, so we have a Zn and Cu electrode. The || represents the salt bridge. To the left of the salt bridge is always the anode, and to the right is always the cathode. So, the anode is zinc and the cathode is copper. You can write two half reactions, recognizing that oxidation happens at the anode and reduction happens at the cathode, and an overall reaction that combines the two
Pt | Fe2+, Fe3+ || Cu2+ | Cu
There is an inert platinum (Pt) anode (it does not participate in redox chemistry) and a copper (Cu) cathode.
The half reactions are:
Anode: Fe2+ → Fe3+ + 1 e–
Cathode: Cu2+ + 2e– → Cu(s)
Overall: 2 Fe2+ + Cu2+ → 2 Fe3+ + Cu(s)
The overall reaction requires electrons to be balanced, so the anode
must be multiplied by 2
Electrolytic Cell Reactivity
• Determine the reactants and products in the electrolysis of molten salts to produce elements at cathode and anode.
o E.g. NaCl → Na at cathode and Cl2 at anode
• Predict which reactants and products complete for electrolysis in
o Mixtures of salts (molten)
o Salt in H2O
Remember to flip the sign from the table to find the
oxidation potentials
Using the table of reduction potentials, be able to explain
• How reduction and oxidation potential are found
• Standard conditions (1M and 1 atm, 298K)
• Reference electrodes (Standard hydrogen electrode at 0.0V)
• Where the strong oxidizing and reducing agents are found. Where famous coinage metals are found. Where
famous electrolysis products are found. Where the elements that prevent rust are found.
Given the two half-cell reactions, find E°cell using
E°cell = E°cathode – E°anode
Nernst equation
Ecell = E°cell − 0.06/n logQ
Q=
Products/Reactants or Right/Left
Q < 1 means
more reactants
Q = 1 means
equal ratio of products:reactants
Q > 1 means
more products
Q = K means
the system is at equilibrium
When E > E°
there are more reactants than products
When E = E°
there are equal products and reactants
When E < E°
there are more products than reactants
When E = 0 or Q=K
the system is at equilibrium (dead battery)
Nernst Equation Calculation
1. Determine cathode and anode then find E°cell
2. Combine half-cells to get unbalanced reaction
3. Balance (no acid/base) to find n and Q
4. Set up equation: E = E° − .06/n logQ
5. Plug in concentrations and solve
Dead Battery Equation
0 = E°cell − 0.06/n logK
A battery is “dead” when it has
0 cell potential, which happens when the cell reaches equilibrium.
n is found as the
lowest common multiple of electrons in the two half-cell reactions
Faraday – Plating Equation
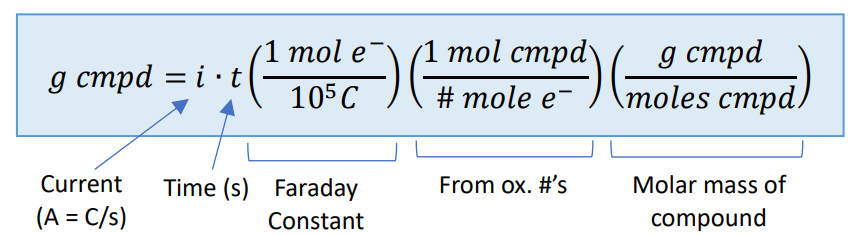
Electrochemical Stoichiometry
For K > 1
product is favored, ∆G is (–), spontaneous reaction
For K = Q
the reaction is at equilibrium and ∆G = 0
For K < 1
reactant is favored, ∆G is (+), nonspontaneous reaction
Be able to convert between K, ∆G°, and E°cell
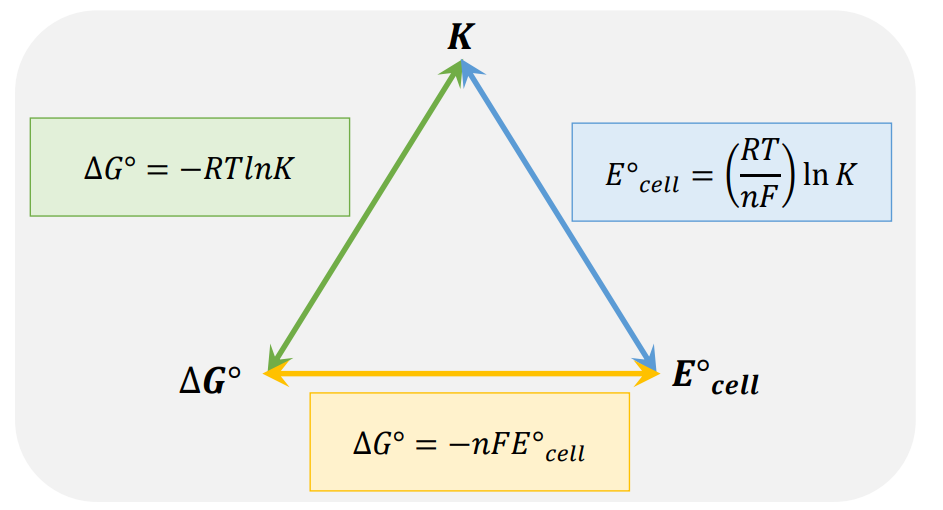
Electrochemistry is just another way of describing thermodynamics. There is a simple equation relating them
ΔG° = −nFE°cell
• The calculation is as simple as it looks. Things to know: n is the lowest common multiple found in balancing a reaction—it will usually be given to you.
• F is the Faraday constant. You will use the approximation 105 C/mole to make the calculation easier.
• Eo is the standard cell potential either given to you or as two half reactions.
• Multiply termstogether and divide by 1000 to convert Eo
cellto Delta Go in kJ
E°cell + means
Delta G° is –
E°cell – means
Delta G° is +
Groups I & II: e.g. Na, Ca, …
React in Cold Water
Other metals: e.g. Al, Zn, Co, Ni, Sn, …
React in Steam (Boiling Water)Acid
Coinage metals: Cu, Ag, Pt, Au
Don’t React in water
Also understand displacement reactions of metals
• The most reactive metals are in the lower right corner (Li is the strongest reducing agent)
• The least reactive metals are in the top right corner (Au is the weakest reducing agent)
• General displacement reaction: M1 + M2X → M2 + M1X in which Metal 1, which is more active, replaces Metal 2, which is less active.
Battery technology pushes boundaries by trying to make the
smallest, least expensive, highest power, longest life, reversible and environmentally safe battery
Secondary batteries are built with consideration given to
minimizing liquid or gas production so that contents of the reaction are better contained in the battery casing for recharging
At current limits with these extreme competing demands, lithium-ion batteries have been known to
catch fire and, for example, currently are not allowed to be shipped as commercial cargo on planes
Parts of a Lithium-ion Battery
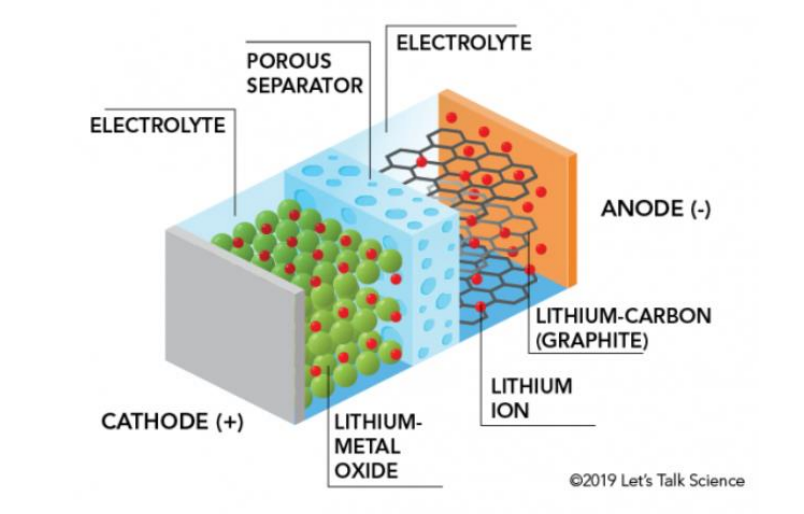
Modern batteries are made with
solids and pastes rather than liquids and gases to avoid losses of material.