MEDS2011 - All topics
1/533
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
534 Terms
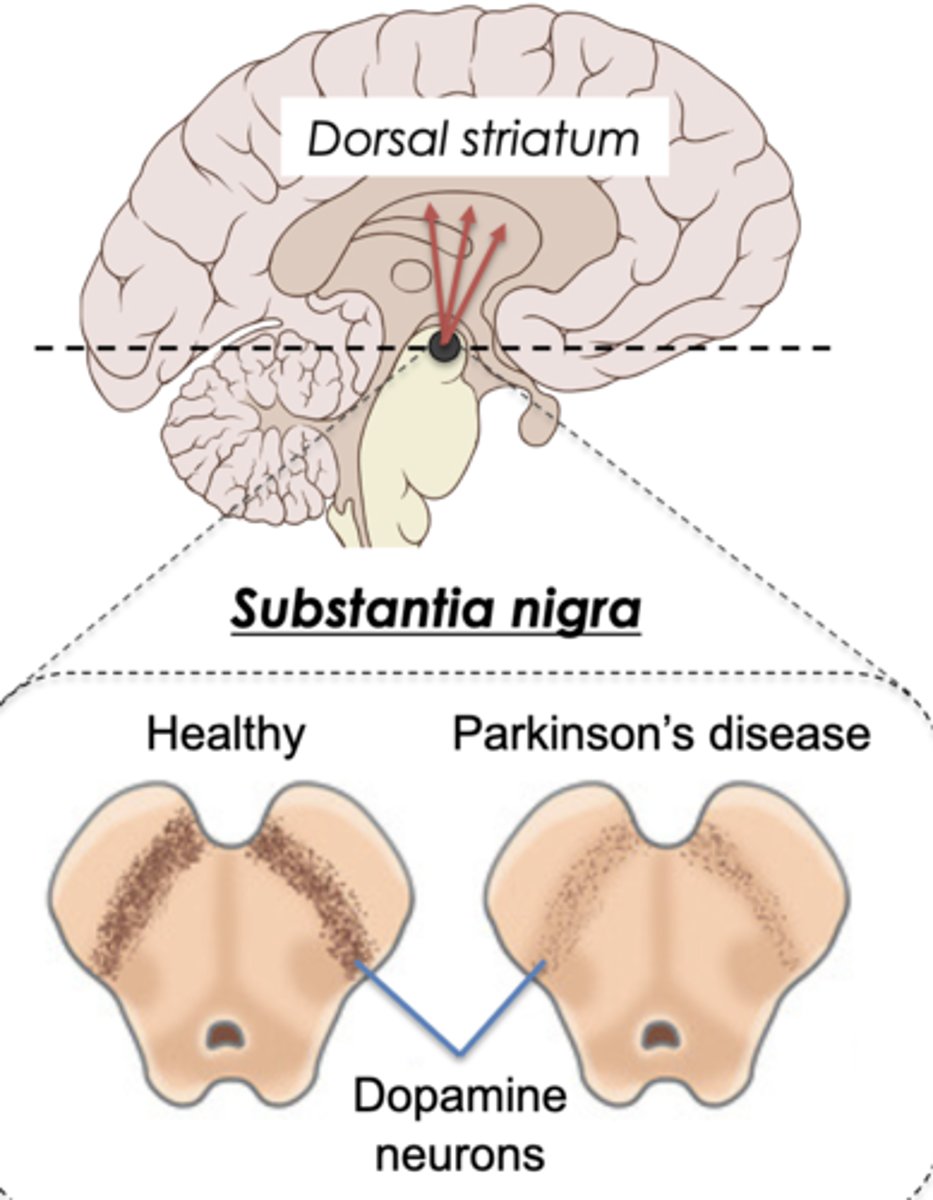
Two primary pathological hallmarks for Parkinson disease
1. Progressive death of dopamine neurons in substantia nigra.
2. Deposition of abnormal forms of protein (α-synuclein, superoxide dismutase 1, tau)
Causation of Parkinson disease
~90% are idiopathic (unknown cause)/ sporadic (scattered) disease
~10% are genetic/familial disease (mutation in gene for α-synuclein protein)
Location of α-synuclein in healthy brain
In synapses
Location of α-synuclein in Parkinson disease brain
α-synuclein become insoluble and deposits in cell bodies, forming Lewy bodies (hallmark of dementia)
Definition of aetiology/etiology
The cause or origin of a disease
What factors are involved in aetiology of Parkinson
Genetic, environmental, and lifestyle factors
What factors increases the risk of Parkinson Disease
Aging, REM Sleep Behaviour Disorder, male sex, exposure to pesticides/solvents/air pollution, genetic susceptibility/mutations, rural living
What factors decreases the risk of Parkinson Disease
Exercise, Mediterranean diet, smoking, coffee/tea
Why do substantia nigra dopamine neurons die?
The changes in the interlinked molecular pathways create a self-perpetuating neurodegenerative cascade.
How can α-synuclein seed amplification assay potentially be used as a biomarker?
Patient blood/CSF sample contain tiny α-synuclein aggregates that stimulate the aggregation of additional α-synuclein. The rate of aggregation is higher in Parkinson patients or healthy people with genes/clinical signs.
What is the basal ganglia?
A group of interconnected regions deep in the brain involved in the regulation of voluntary movements.

Definition of biomarker
A substance /trait used as an indicator of a biological state, such as a disease
Definition of burden of disease
Term used in public health for measuring the impact of a disease on a population
Definition of Bradykinesia and Akinesis
Bradykinesia: slowness of movement
Akinesis: lack of movement
What are Lewy bodies?
A mature form of abnormally aggregated insoluble proteins, including α-synuclein, in Parkinson brain.
Definition of preclinical/prodromal disease
An early disease stage before the disorder is clinical recognisable
REM Sleep Behaviour Disorder (RBD)
A sleep disorder in which people move and 'act out their dreams' during REM
What is spina bifida?
- A neurodevelopment disorder and one of the most common of the neural tube defects (NTDs).
- Errors in the development of the caudal spinal cord in utero
Rostral (towards oral or nasal region) neural tube closure failure causes what disease?
Anencephaly ("no brain" - fatal)
Caudal neural tube closure failure causes what disease?
Spina bifida ("split spine")
Formation of spina bifida
- Vertebral arches fail to develop, leading to maldevelopment of vertebral column, spinal cord and nerves and associated muscles
- A sac-like cyst may protrude from the spine
Defects can be "open" or "closed" (skin covered)
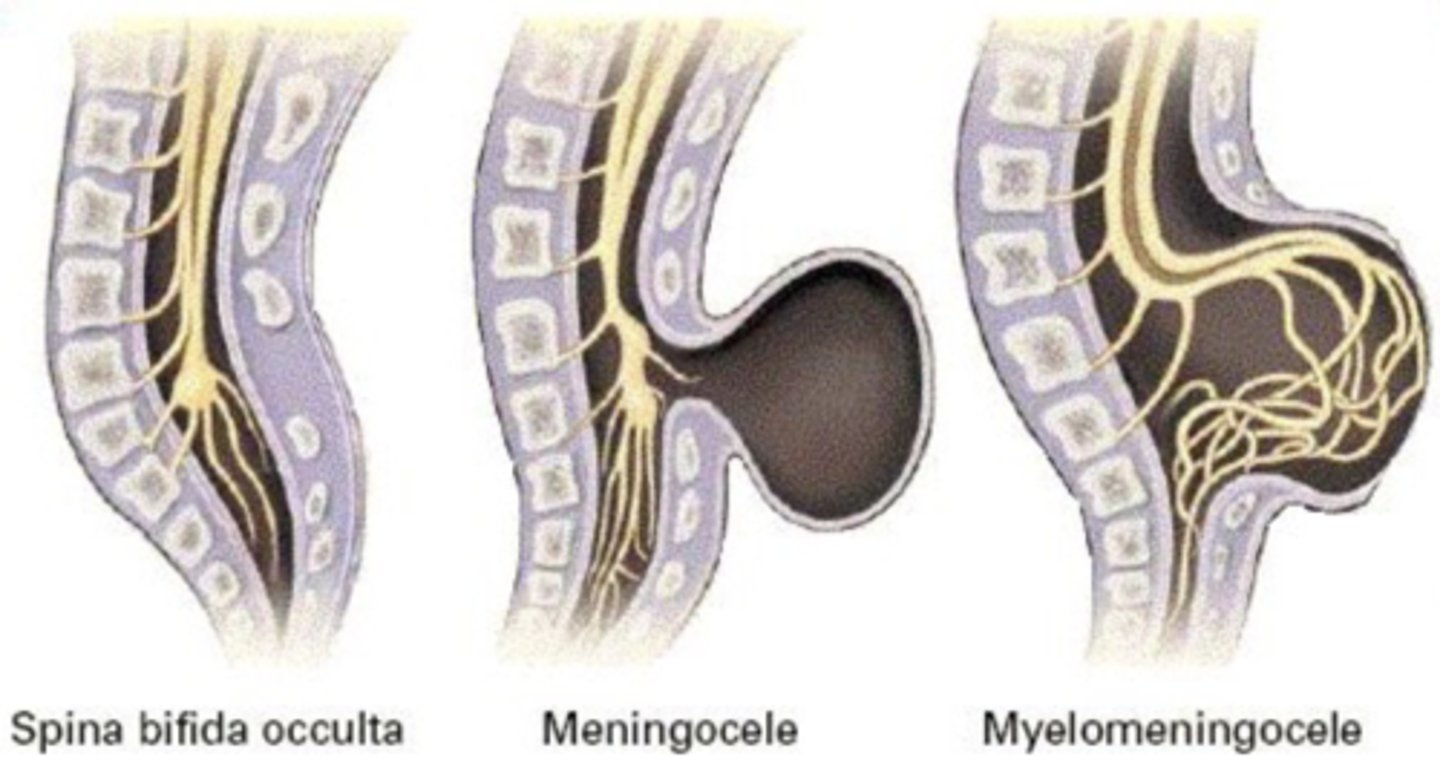
What is spina bifida occulta?
- Mildest form, one or more vertebrae (usually L5 and S1) are not closed but no protrusion of spinal cord.
- Cutaneous stigmata at lesion level
- May be associated with cyst in spinal cord or tethered cord syndrome
- Often associated with anorectal and/or urogenital malformations, bowel, bladder, and sexual dysfunction.
What is Meningocele?
Sac-like cyst of meninges filled with spinal fluid, usually with no involvement of spinal cord, but possible spinal abnormalities.
What is Myelomeningocele?
- Protrusion of meninges and spinal cord through vertebral defect.
- 80% in lumbar and sacral regions
- Involvement of neural tissue with paralysis of limbs and abdominal muscle, loss of sensation and incontinence
- Most severe and common form
- Surgery usually required, prior to or soon after birth
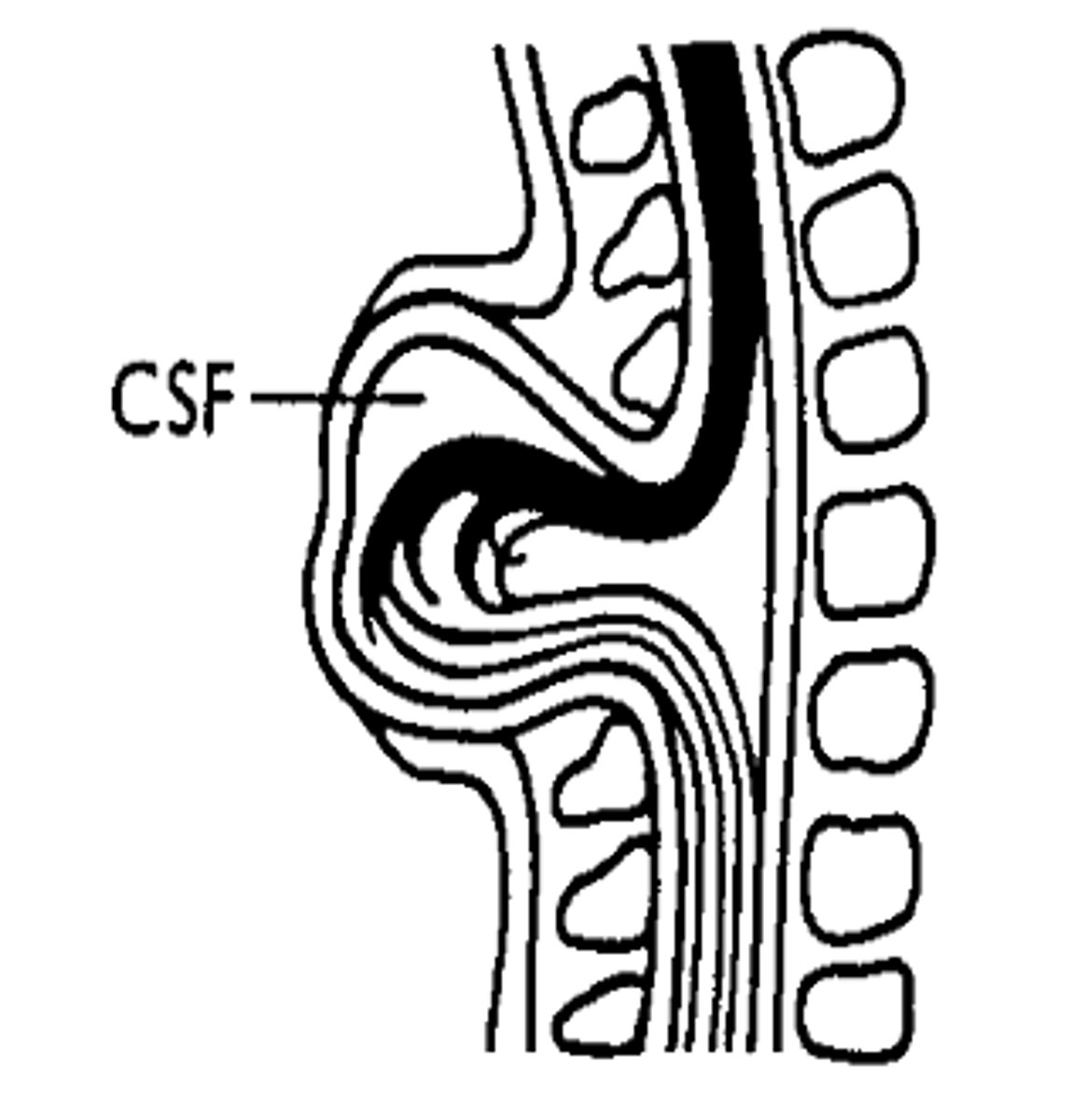
Three possible complications of myelomeningocele: Chiari II malformation
1. Extrusion of cerebellum and brianstem below foramen magnum
2. Variable effects: sensory and motor symptoms through the paralysis, brainstem damage and hydrocephalus
3. May require prenatal surgical decompression of brain as an emergency
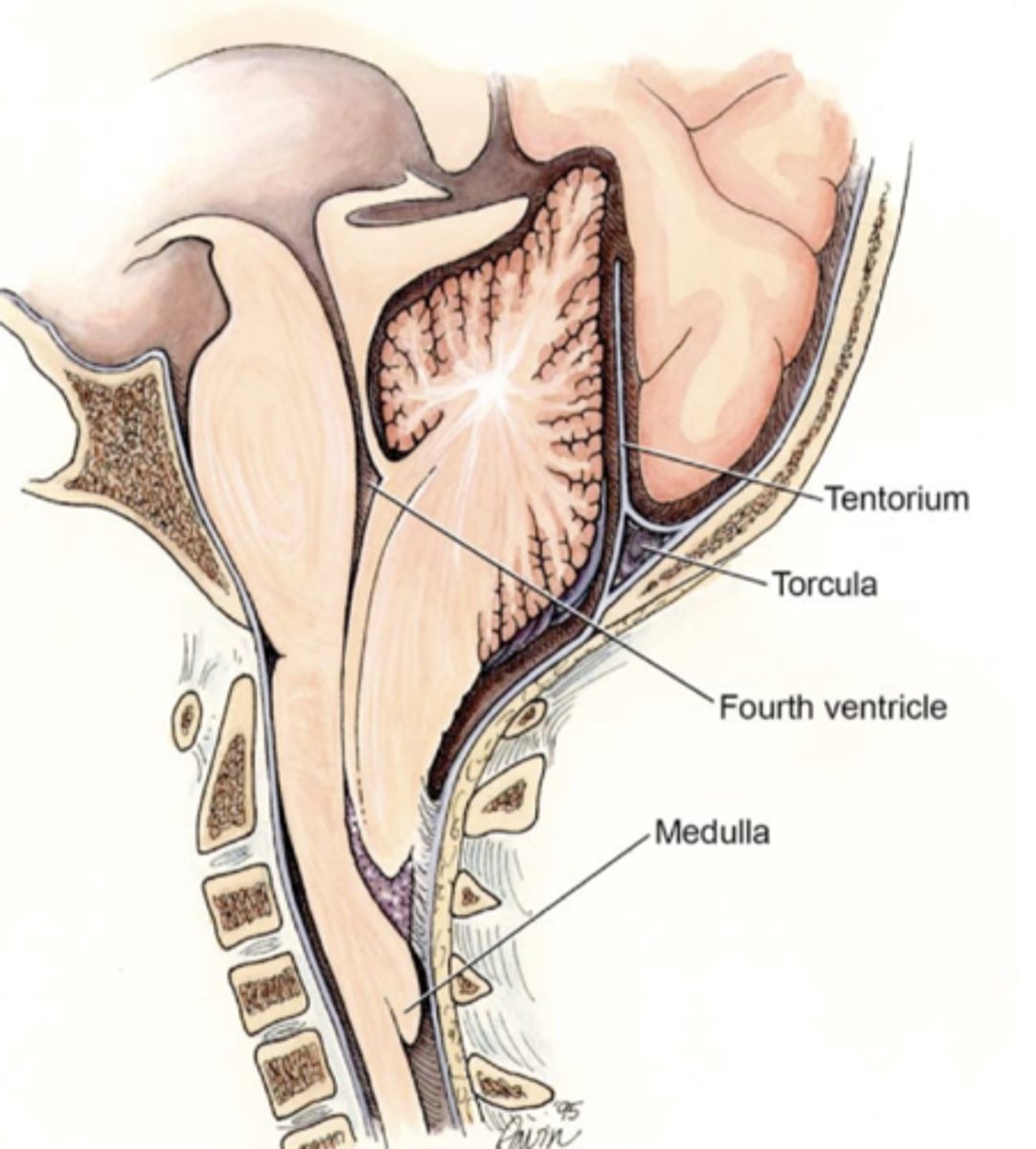
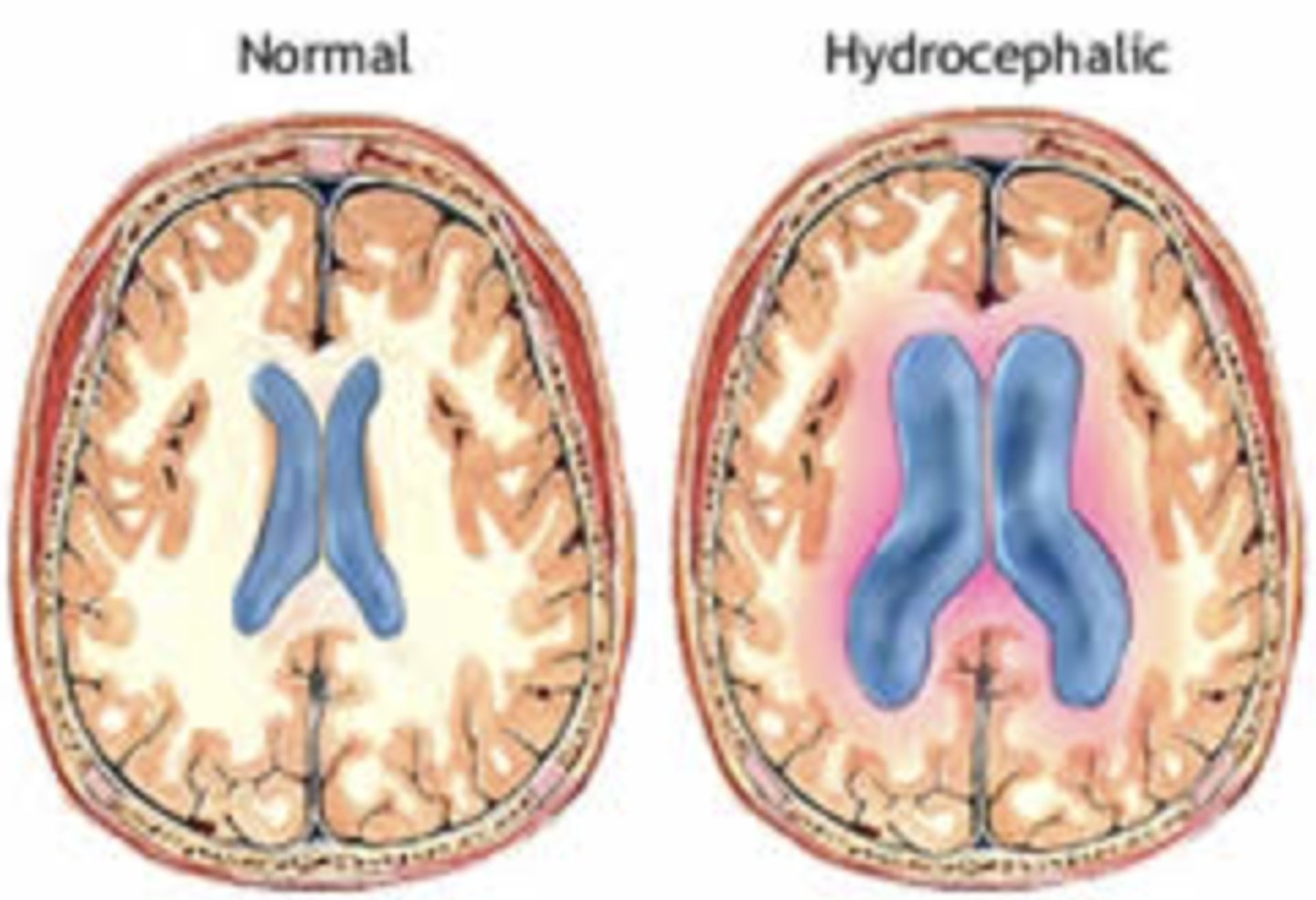
What is hydrocephalus?
- "water head"
- Obstruction of CSF flow leads to enlargement of ventricles, compression of brain tissue and expansion of the head
- Severity associated with poorer neurological outcomes
How can spina bifida be diagnosed in utero?
Through ultrasound imaging in the first trimester or by measuring maternal serum levels of alpha-fetoprotein.
What determines the functional effects of spina bifida in a patient?
The level of the spinal cord lesion.
What are some common functional effects of spina bifida?
Paralysis, numbness, bladder/bowel dysfunction, sexual dysfunction, and cognitive impairment.
What is the key to good management of spina bifida?
Early diagnosis followed by proactive, lifelong, multidisciplinary specialty care.
Which specialists are typically involved in the management of spina bifida?
Urology, neurosurgery, orthopaedics, physical medicine and rehabilitation, psychology, and assistive technology services.
What is the average life expectancy of individuals with spina bifida?
Around 50 years
What are common causes of death in individuals with spina bifida?
CNS dysfunction, cancer, urological disease, and sepsis.
Some causes of spinal bifida
- Mostly sporadic but genetic predisposition likely; effect of sex and ethnicity
- Environmental risk factors including maternal health diabetes, obesity, hyperthermia, drug exposure, substance abuse, air pollution, poor nutrition, low vitamin B12 and B9, alcohol, smoking
- Folate found critical for neural tube closure during wks 3-6 of embryogenesis
Definition of agenesis
Absence of, or incomplete, development of an organ or tissue
Definition of anencephaly
A lethal defect in brain development resulting in the absence of most of the brain and skull.
What is folate?
Vit B9, nutrient gained from diet essential for cell growth and reproduction
What is folic acid?
A synthetically produced dietary supplement which is converted to folate in the body.
What is foramen magnum?
The large opening at the base of the skill through which the spinal cord passes

Definition fo heterotopia
A normal tissue or organ present at an abnormal location in the body
What is meninges?
The three membranes which enclose the brain and spinal cord.
What is polymicrogyria?
A rare genetic disorder characterised by structural abnormalities in the cortex of the brain
What is syrinomyelia?
a disorder characterised by a fluid-filled cyst/s in the spinal cord which compresses the nervous tissue and can lead to paralysis
What is tethered cord syndrome?
Disorders associated with abnormal limitations in the movement of the spinal cord.
How effective are antiepileptic drug treatments?
Only 60-70% patients are seizure free.
Three ILAE classification of seizure types
Focal onset
Generalised onset
Unknown onset
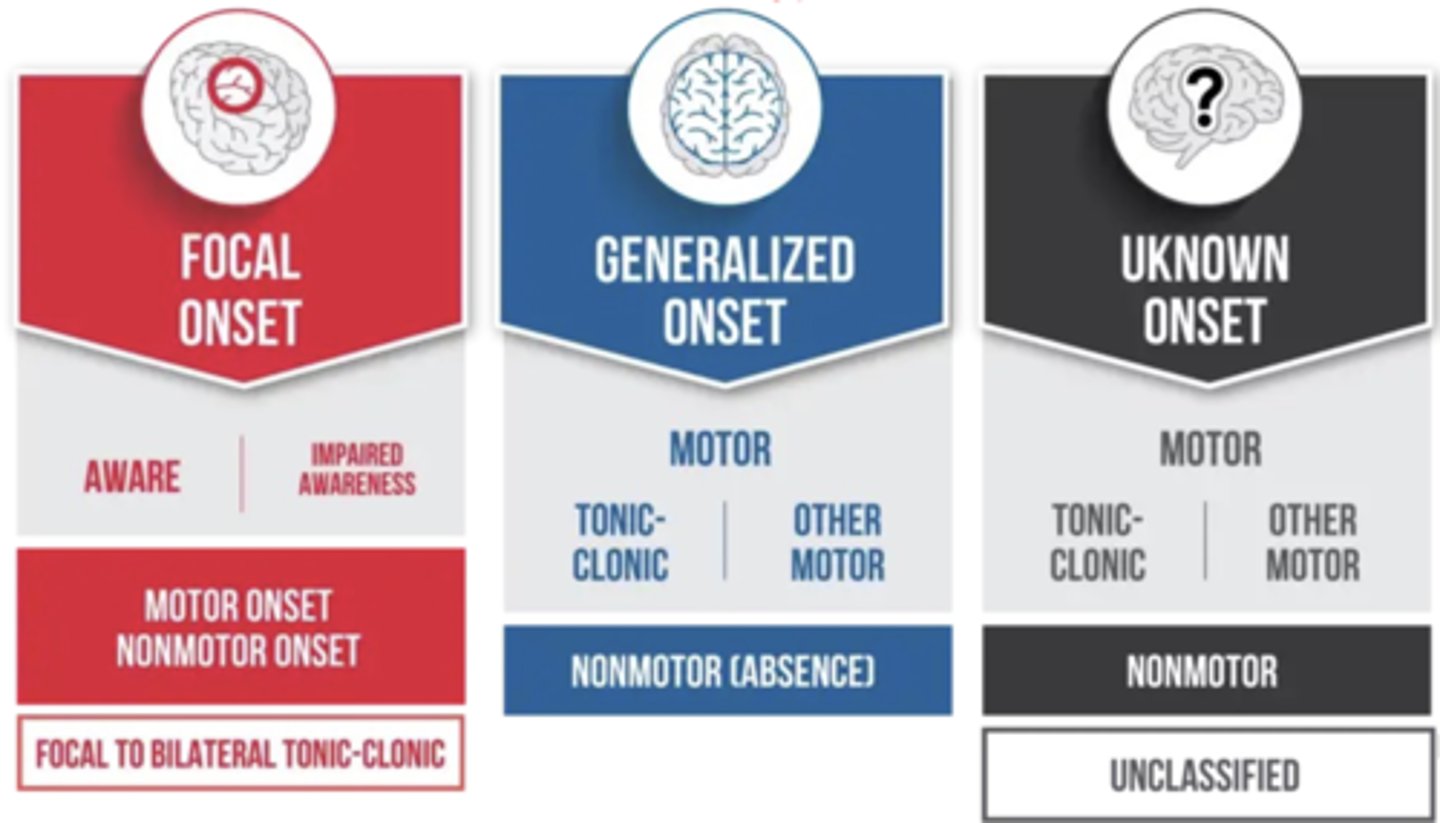
How can seizure types be differentiated and identified?
Via electroencephalogram (EEG) seen as spikes and waves
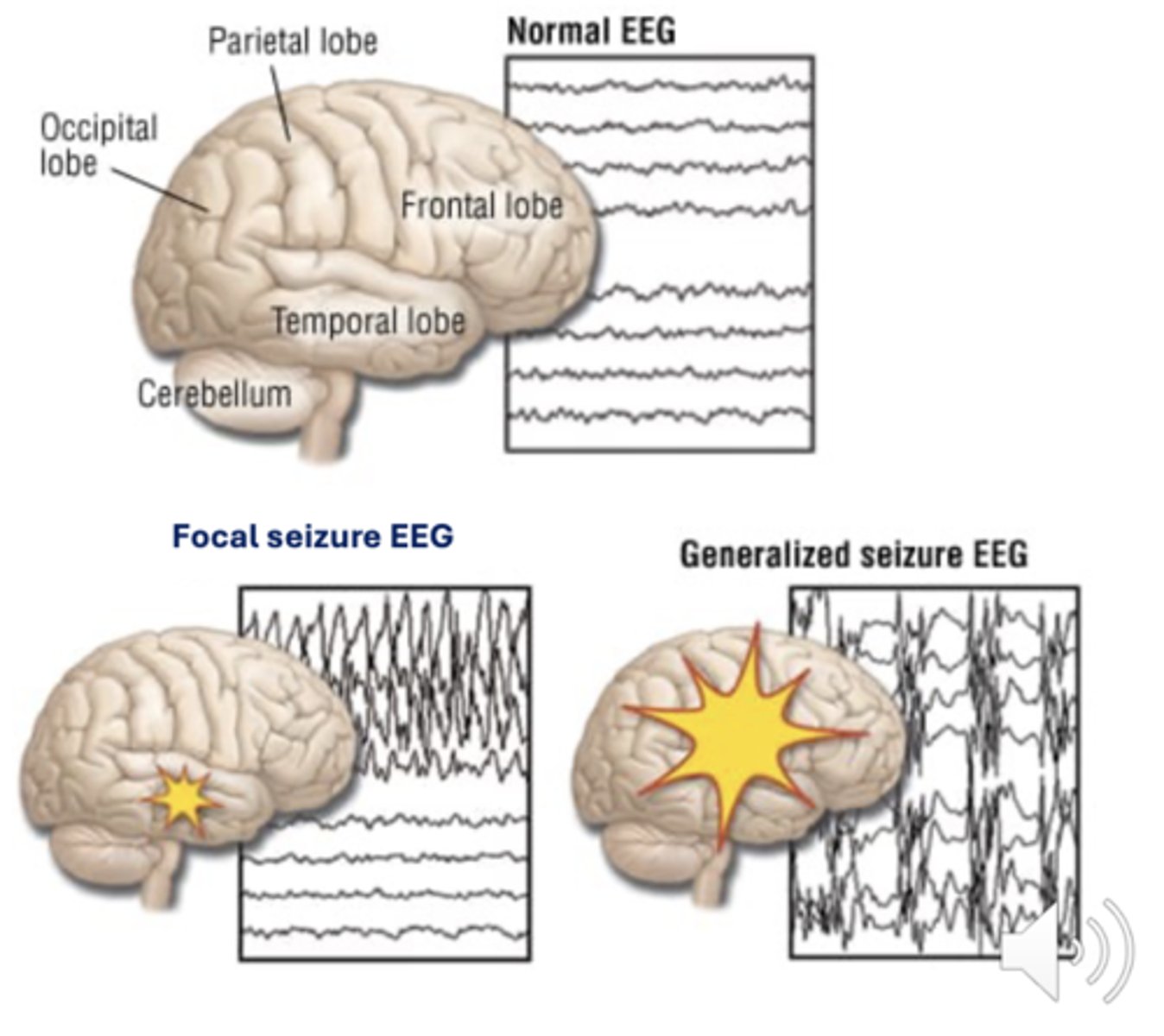
What is status epilepticus, and how does it present?
Status epilepticus is a condition where a seizure is prolonged or seizures occur in close succession without recovery in between. It can occur with any seizure type and may be convulsive or non-convulsive.
Why is status epilepticus considered a medical emergency?
Because the longer a seizure lasts, the less likely it is to stop on its own, increasing the risk of long-term damage or death. Prompt medical attention is essential.
How do the origin and spread of seizures influence epilepsy syndromes?
Depending on where seizures begin and how they spread in the brain, different symptoms beyond seizures can occur, resulting in distinct epilepsy syndromes.
What characterises epilepsy syndromes?
A set of clinical features, signs, and symptoms that occur in addition to seizures and identified in infancy or early childhood.
Three examples of epilepsy syndromes
1. Myoclonic epilepsy in infancy (MEI)
2. Genetic epilepsy with febrile seizures plus spectrum (GEFS+)
3. Syndromes with Developmental and Epileptic Encephalopathy (DEE)
Causes of epilepsy
Genetics, head injury, stroke or brain tumour, encephalitis, drugs and medication, cysticercosis, developmental conditions, brain injury before birth
70-80% cases are due to genetics (inherited or spontaneous)
What are the potential benefits and limitations of genetic testing in epilepsy?
Genetic testing can help identify the cause of epilepsy, support accurate diagnosis, guide personalised treatment, inform family members of their risks, and avoid contraindicated medications.
The excitatory and inhibitory neurotransmitters that maintain brain homeostasis
Inhibitory: GABA
Excitatory: Glutamate
What type of seizure involves sustained rhythmical jerking movements?
Clonic seizures
What seizure type causes muscles to become weak or limp?
Atonic seizures
What type of seizure causes muscles to become tense or rigid?
Tonic seizures
What seizure type involves brief muscle twitching?
Myoclonic seizures
What are epileptic spasms?
Seizures where the body flexes and extends repeatedly.
What non-motor symptoms may occur with absence seizures?
Staring spells or brief twitches (myoclonus), often affecting the eyelids or specific body parts.
Definition of osteoporosis
A progressive disease that weakens bones, increasing the risk of fractures.
Briefly explain the process of bone remodelling
- Events stimulates the activation phase
- Osteoclast precursors are recruited and are activated (i.e. by RANKL) and attached to the bone surface
- Osteoclasts degrade the bone and liberate bone matrix components, then undergo apoptosis
- macrophages clear away the debris
- osteoblasts deposit osteoids to fill the cavity, and then mineralise it after
- Some osteoblasts become trapped and differentiate into osteocytes. Others undergo apoptosis or become bone-lining cells.
Origin and function of osteoclasts
- derived from haematopoietic pre-cursor cells
- resorb bone matrix by secreting acid to demineralise and proteases to breakdown collagen matrix
- minerals and fragments of bone matrix type I collagen are released into the circulation during matrix degradation
Origin and function of osteoblasts
- derived from mesenchymal lineage
- form organic matrix (osteoid) and then mineralise it by incorporating hydrozyapatite. Then differentiate into osteocytes and embedded in bone matrix.
- Secrete various proteins during matrix formation
Function of osteocytes
- Forms a canalicular network between each other
- Can sense mechanical forces
- Respond to load in tissue
- Releases factors which increase (RANKL) or decrease (OPG) osteoclast formation and activity
- Release factors which inhibit (sclerostin) osteoblast differentiation and acitivity
What happens to the balance of of osteoclast and osteoblast in osteoporosis?
Osteoclast activity increases, resulting in more bone resorption than bone formation.
What hormones monitor calcium release and retrieval?
Calcitonin & parathyroid hormone
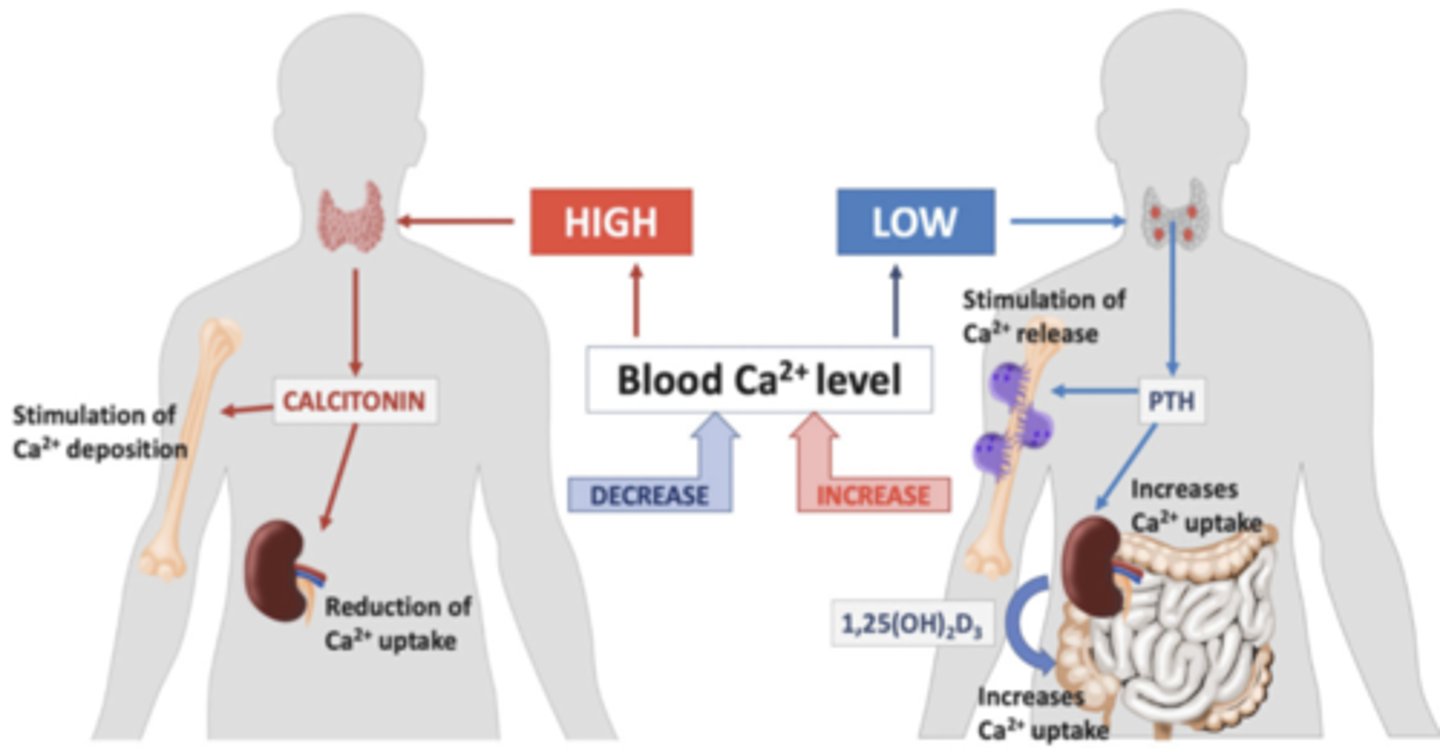
Calcium in osteoporosis
Low calcium intake increases osteoporosis risks.
Calcium absorption decreases with age.
Recommended dietary calcium intake for adults in Australia
Male: <70 years need 1000mg/day; >70 years need 1300ng/day
Female: <50 years need 1000mg/day; >50 years need 1300ng/day
Role of Vitamin D in osteoporosis
- Vit D deficiency increases risk of osteoporosis
- Older adults are at risk of Vit D deficiency
- reduced capacity to produce or absorb vit D
- Reduced sun exposure
- Low dietary intake of vit D
- Medication that depletes vit D
Recommended dietary vit D intake for adults in Australia
- 5 ug/day (19-50 years)
- 10 ug/day (51-70 years)
- 15 ug/day (>70 years)
How do estrogens, particularly estradiol (E2), contribute to bone homeostasis?
Estrogens, especially E2 (estrodiol), help regulate bone homeostasis by directly and indirectly affecting osteoblasts and osteoclasts.
How is estradiol (E2) produced in males?
In males, E2 is produced from testosterone through conversion by the aromatase enzyme.
What happens to estrogen (E2) levels and bone health in women after menopause?
Ovarian E2 production decreases, leading to rapid bone loss of about 3-5% per year for 5-10 years.
What factors influence the onset and severity of osteoporosis after menopause?
Genetic factors, exercise, BMI, smoking, and nutrition.
Why are men with low testosterone at risk of osteoporosis?
Low testosterone reduces estradiol levels, which is important for bone health, increasing the risk of osteoporosis.
What is the effect of E2 and testosterone deficiency on bone remodeling?
It increases bone remodeling, osteoclastogenesis, osteoblastogenesis, bone resorption, and bone formation—but bone resorption exceeds bone formation, leading to bone loss.
Musculoskeletal changes with aging that may cause sarcopenia
Muscle mass, Grip strength, Gait (walking) speed
Definition of sarcopenia
Progressive and generalised skeletal muscle disorder involving the accelerated loss of muscle mass and function.
Adverse outcomes associated with sarcopenia
Falls, functional decline, frailty, and mortality
Diagnosis of Sarcopenia
Involves measuring at least two parameters related to:
- muscle mass
- muscle strength (e.g. grip strength)
- Physical performance (e.g. gait speed)
Common causes of Sarcopenia
- Nutritional
- Inactivity
- Diseases
- Iatrogenic (caused by medical treatment)
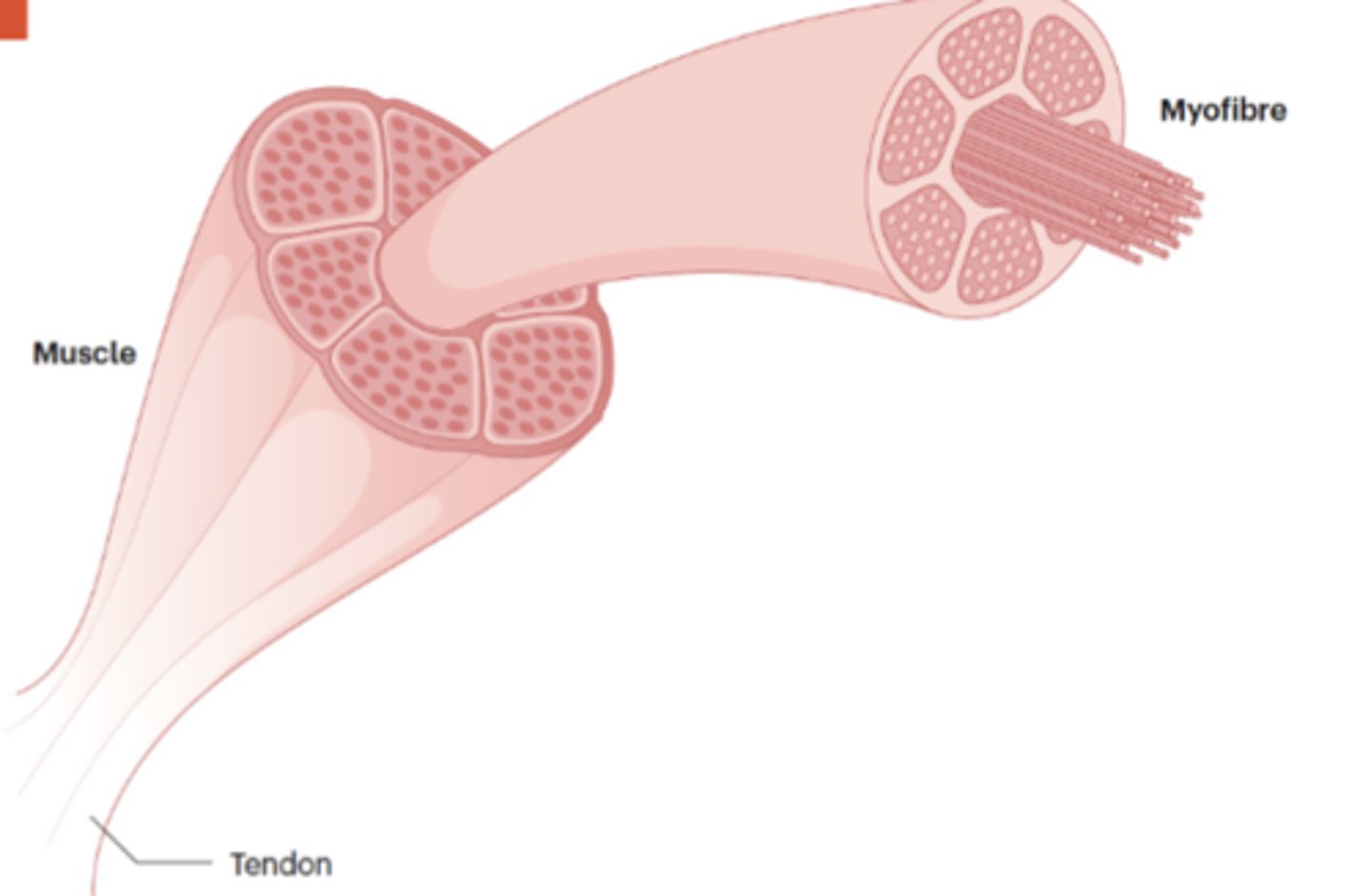
What are skeletal myofibers composed of, and how are they organized?
Skeletal myofibers are large multinucleated cells composed of thick (myosin) and thin (actin) filaments organized into sarcomeres.
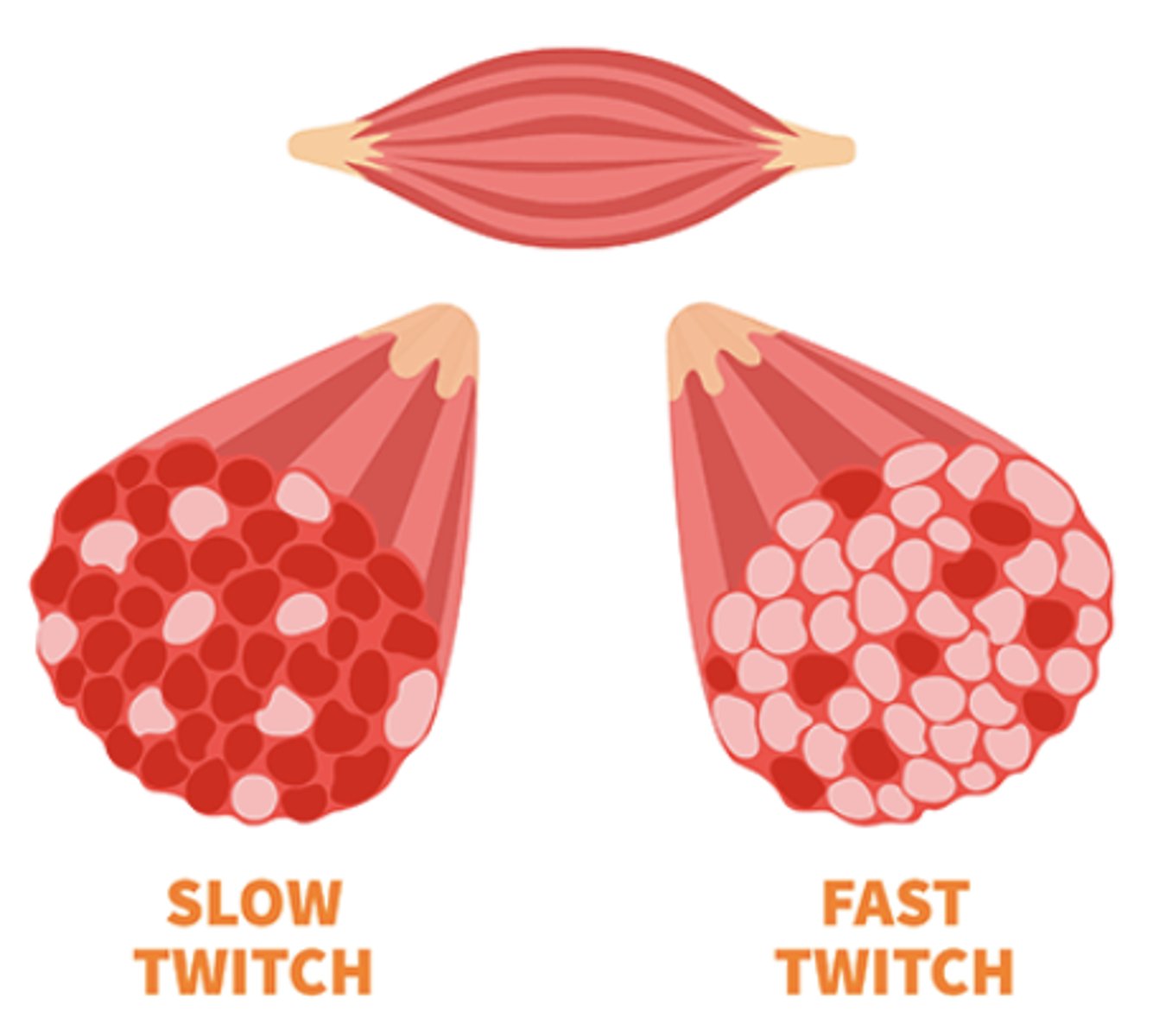
What are the two main types of skeletal muscle fibers, and how do they differ?
Skeletal muscles contain slow-twitch (type I) and fast-twitch (type II) fibers, which differ in contraction speed, metabolic pathways, and response to muscle damage.
Describe the myofibril changes in sarcopenic muscles
- Reduction in size and number of myofibers
- Transition of muscle fibres from type I (slow) to type II (fast) myofibers
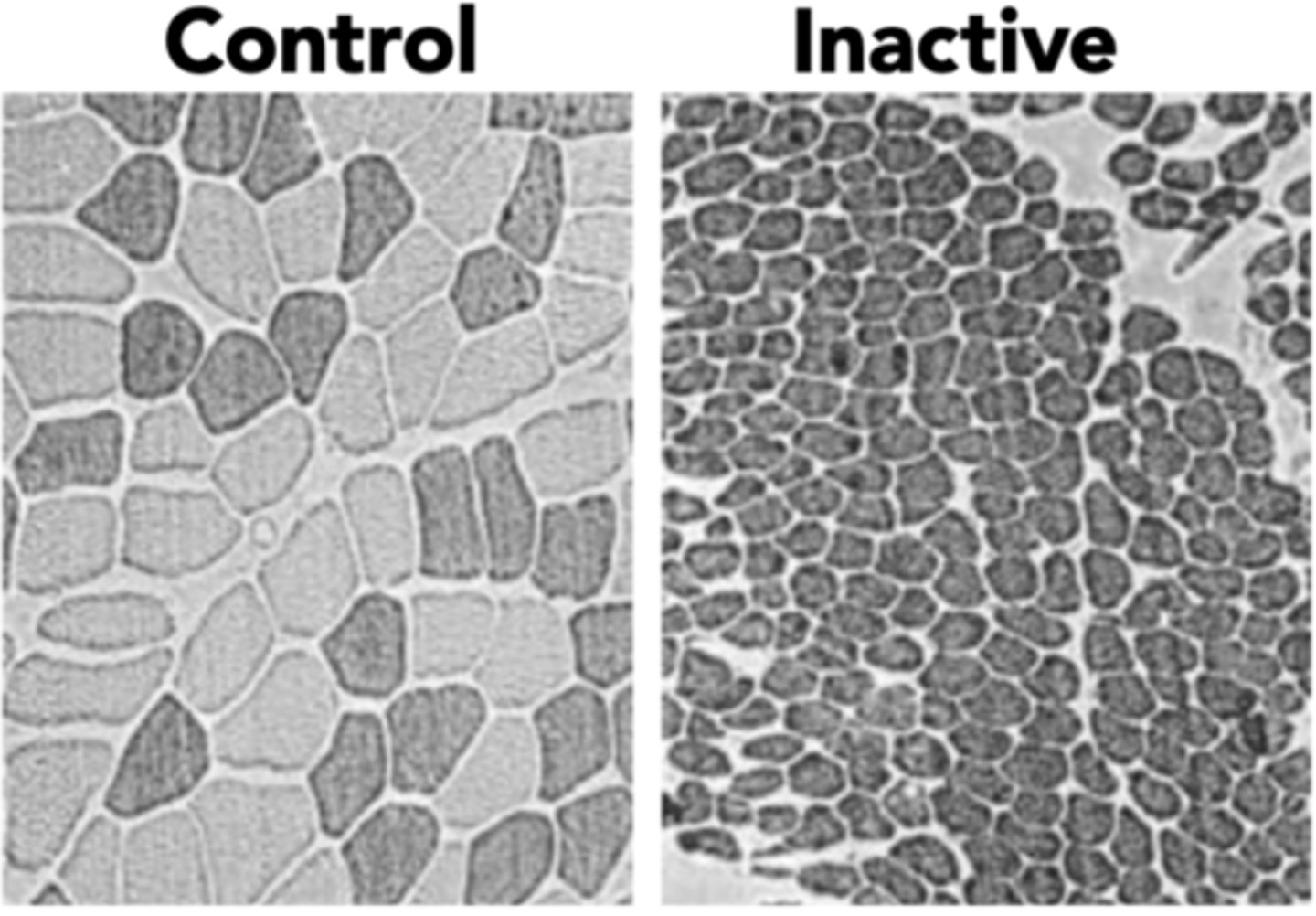
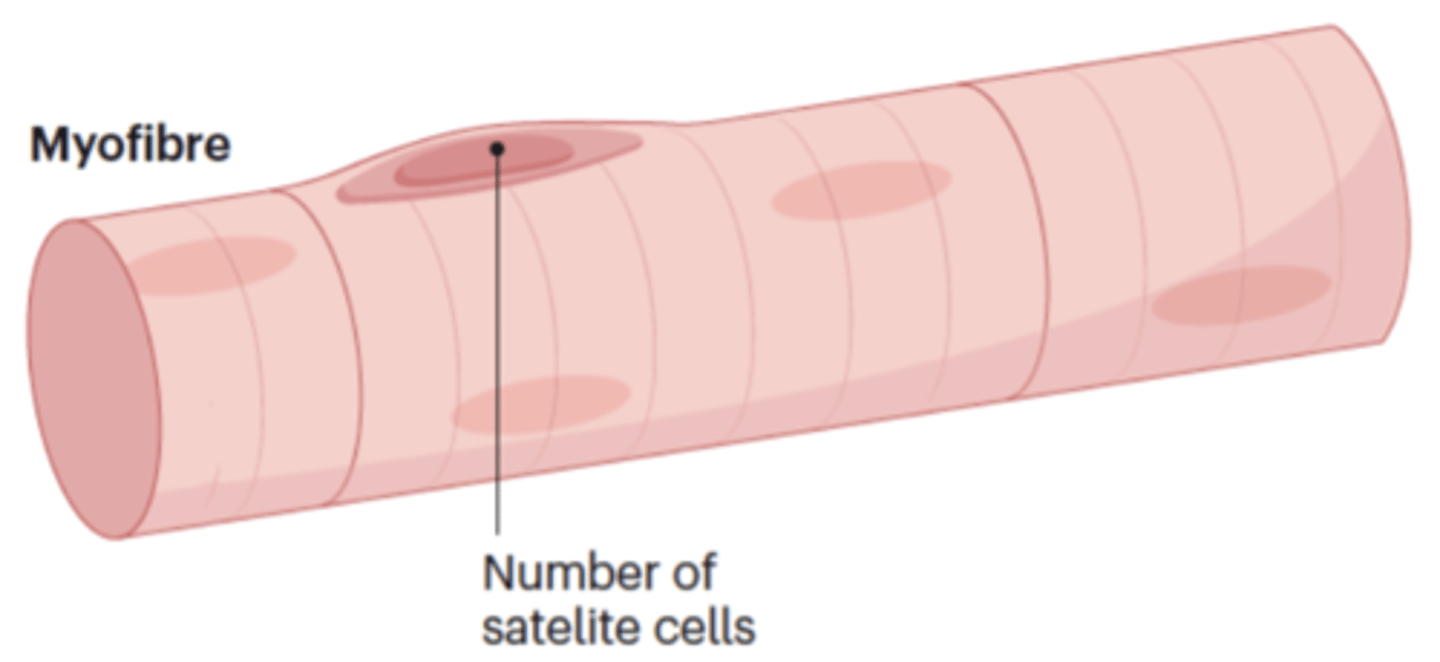
Describe changes in satellite cells in sarcopenic muscles
Satellite cells reduced, that are for muscle maintenance and repair, in type II (fast) muscle fibres
Describe changes in fat distribution in sarcopenic muscles
Fat infiltration due to a decline in energy expenditure from decreased physical activity
How does menopause affect sarcopenia and muscle function in women?
Sarcopenia becomes more common after menopause, likely due to hormonal decline. Oestrogen helps with muscle strength and force generation but not with maintaining muscle size.
What causes sharp declines in testosterone (T) and estradiol (E2) levels in aging men?
Though T levels vary with age, sharp declines can occur due to orchiectomy, hormone-suppressing medications, or testicular injuries.
What are the effects of testosterone (T) deprivation on muscle and mobility in men?
T deprivation leads to muscle atrophy, increased fat mass, reduced muscle strength, and changes in walking and balance—raising the risk of falls.
4 causes of bone fractures
Osteoporosis, arthritis, sarcopenia, neurological conditions - Parkinson's
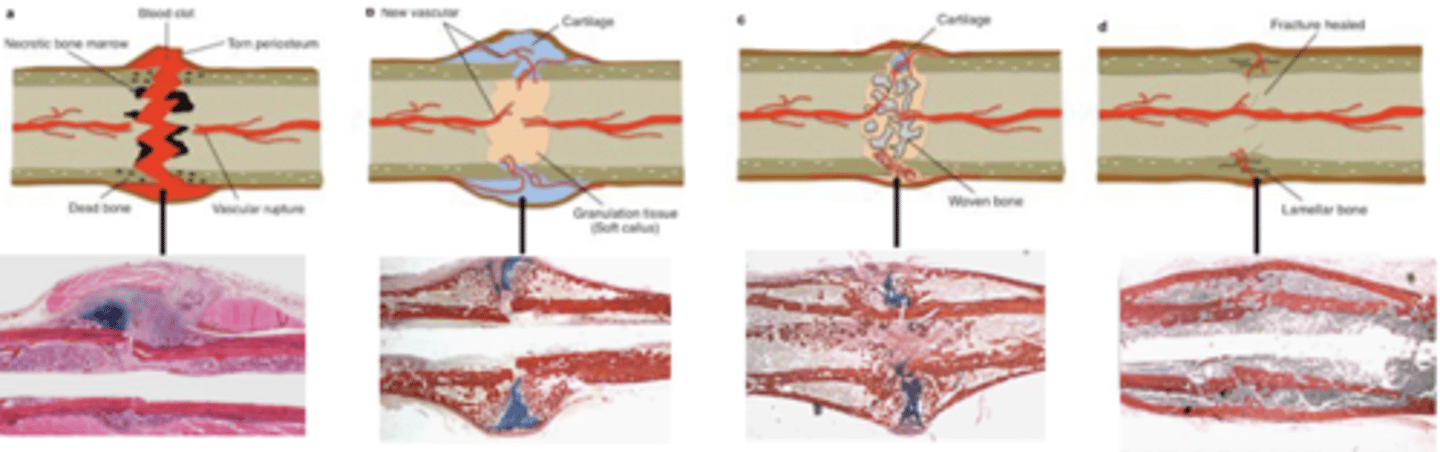
4 steps in endochondral fracture repair
1. Blood clot formation (early stabilisation, immune cell infiltration-granulation tissue)
2. soft callus formation (stabilisation but some movement, cartilage (soft) callus replaces blood clot)
3. Hard callus formation (Bridged/union, vascularisation of callus, cartilage callus replaced with woven bone)
4. Hard callus remodelling (osteoclasts remodel woven bone into mature cortical bone, return to original shape)
Local tissue level causes of impaired bone fracture healing
Restricted blood flow, infection, stability
Systemic level causes of impairing bone fracture healing
Poor nutrition, smoking, diabetes, medications, age and co-morbidities
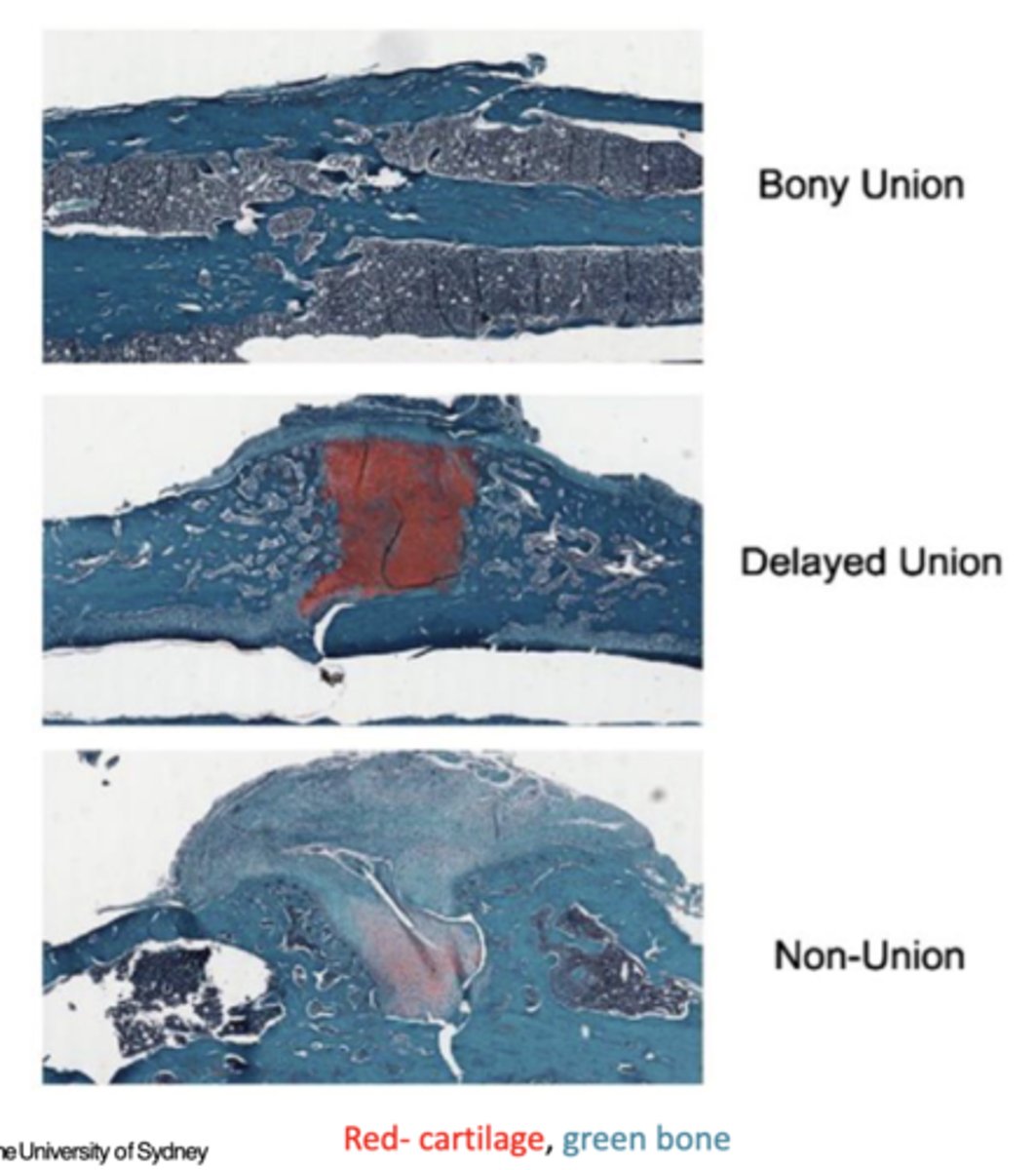
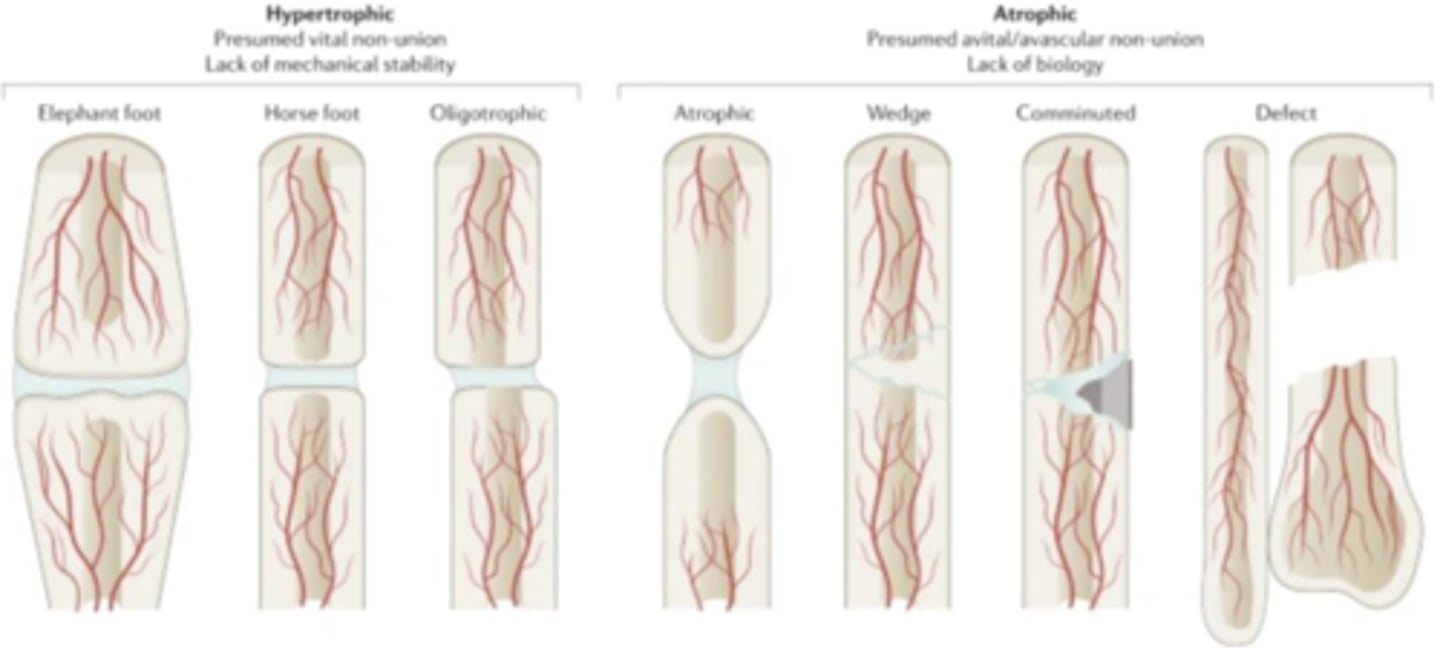
Difference between hypertrophic and atrophic bone healing
Hypertrophic: presumed vital non-union, lack of mechanical stability
Atrophic: presumed avital/avascular non-union, lack of biology
The impact of osteoporotic fractures on quality of life in 4 aspects
Physical health: chronic pain, reduced mobility, and long term disability
Emotional well-being: pain and physical lead to emotional distress
Overall health status: lower overall health-related quality of life measures
Lifespan: mortality in first year following a hip fracture is 20%
How does osteoarthritis (OA) impact individuals and society?
OA causes more pain, fatigue, disability, and activity limitations than in non-OA peers; 25% can't perform daily tasks, 30% have co-morbidities, and it increases cardiovascular disease risk by 50%.
Why is a joint considered a functional organ?
A joint consists of different tissues that work together to enable movement, bear loads with low friction, adapt to physiological demands, and include neural/proprioceptive functions.
How does osteoarthritis (OA) affect the joint as an organ?
OA is considered joint "organ failure" and involves active biological processes that disrupt the function of joint tissues.