Chem 2 Midterms Reviewer
1/124
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
125 Terms
States of matter
The fundamental difference between _____ is the distance between particles.
Distance between particles
The fundamental difference between states of matter
Kinetic energy of the particles
Strength of attractions between particles
The state a substance is in at a particular temperature and pressure depends on two antagonistic entities:
Temperature
Pressure
The state a substance is in at a particular (1) and (2) depends on two antagonistic entities:
Kinetic energy of the particles
Strength of attractions between particles
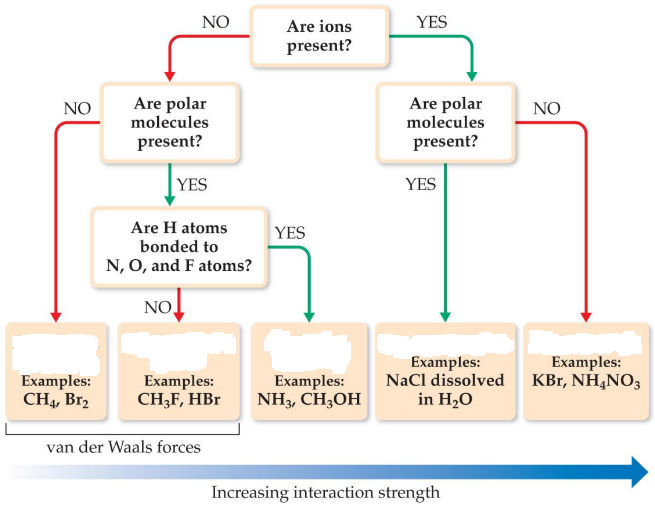
Intermolecular forces
Weaker than intramolecular forces
They are strong enough to control physical properties such as:
boiling point
melting point
vapor pressure
viscosities
As a group, they are referred to as van der Waals forces
Van der Waals forces
Intermolecular forces as a group
Dipole-dipole interactions
Hydrogen bonding
London dispersion forces
H o L D
The 3 van der Waals forces
London dispersion forces
van der Waals force
a.k.a. dispersion forces
Attractions between an instantaneous dipole and an induced dipole
Present in all molecules, ignoring polarity
Polarizability
The tendency of an electron cloud to distort
The tendency of molecules to generate induced electric dipole moments when subjected to an electric field
Shape of the molecule
Molecular weight
The 2 factors that affect dispersion forces
Stronger
Increased surface area
Long, skinny molecules (e.g., n-pentane) tend to have (1) dispersion forces because of (2)
Directly proportional
⬆ Molecular weight = ⬆ Dispersion forces
Larger atoms have larger electron clouds, easier to polarize
The relationship between molecular weight and dispersion forces
Why?
Dipole-dipole interactions
van der Waals force
Molecules that have permanent _____ are attracted to each other
The positive end (δ+) of a molecule is attracted to the negative end (δ-) of the other
Only important when the molecules are close to each other
Polarity
Directly proportional
A factor that affects dipole-dipole interactions
The relationship of this factor to dipole-dipole interactions
Hydrogen bonding
van der Waals force
The dipole-dipole interactions experienced when _____ is bonded to N, O or F are unusually strong
Arises in part from the high electronegativity of N, O and F
The _____ nucleus is exposed
Ion-dipole interactions
Not a van der Waals force
Important in solutions of _____
Its strength makes it possible for _____ substances to dissolve in polar solvents
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Ion-dipole forces
Ionic bonding
LD HI I
Increasing order of IMFA
Boiling point
Melting point
Viscosity
Surface tension
Capillary action
5 liquid properties affected by IMFA
Boiling point
Liquid property affected by IMFA
The temperature at which a liquid boils
The temperature at which its vapor pressure equals the atmospheric pressure
Melting point
Liquid property affected by IMFA
The temperature at which it changes state from solid to liquid
Directly proportional
The relationship between the boiling and melting points of a substance with its IMFA
Viscosity
Liquid property affected by IMFA
Resistance of a liquid to flow
Related to the ease with which molecules can move past each other
Directly proportional
Inversely proportional
The relationship of viscosity to IMFA
The relationship of viscosity and temperature
Surface tension
Liquid property affected by IMFA
Water acts as if it has a “skin” on it due to extra inward forces on its surface
Cohesive forces
IMFA that bind similar molecules to one another (e.g., H2O < Hg)
Adhesive forces
IMFA that bind a substance to a surface (e.g., H2O > Hg)
Capillary action
Liquid property affected by IMFA
The rise of liquids up narrow tubes
Adhesion + cohesion
Vapor pressure
At any temperature, some liquid molecules have enough energy to escape the surface and become gas
Directly proportional
The relationship of vapor pressure and temperature
Directly proportional
The relationship of vapor pressure and pressure
Inversely proportional
The relationship of vapor pressure and IMFA
Volatility
A substance’s degree to transition from a liquid/solid state to a gaseous state under specific temperature and pressure conditions
Inversely proportional
The relationship of volatility and IMFA
Normal boiling point
The temperature at which its vapor pressure is 760 torr or 1 atm
Metallic
Ionic
Covalent-network
Molecular
MMIC
4 general types of solids
Crystalline solids
Atoms in solids that are arranged in a very regular pattern
Amorphous solids
Atoms in solids that have a distinct lack of order in their arrangement
Crystal lattices
Unit cells/tiles
One can deduce the pattern in a crystalline solid by thinking of the substance as a (1) of repeating shapes formed by the atoms in the crystal
The individual shapes of the (1) form (2) that must fill the entire space of the substance
Cubic
Tetragonal
Orthorhombic
Rhombohedral
Hexagonal
Monoclinic
Triclinic
CORTT H&M
7 basic 3D lattices
Close packing
The atoms in a crystal _____ as close together as they can based on the respective sizes of the atom
(Metal) Alloys
Combinations of 2 or more elements, mostly metals
Substitutional alloy
Interstitial alloy
2 types of metal alloys
Substitutional alloy
Type of metal alloy
A 2nd element takes the place of a metal atom
Interstitial alloy
Type of metal alloy
A 2nd element fills a space in the lattice of metal atoms
Metallic bonding
Bond between metals
Form large groups of atoms that share electrons among them
Metal = a group of cations suspended in a sea of electrons
Dense
High melting point
Good electrical conductor
Good heat conductor
Malleable
Ductile
Lustrous
DED HM LM
7 properties of metallic solids
Ionic solids
Type of solid
The lattice comprises of alternately charged ions
Quintessential crystals
Opposite
Like
The different sized-ions in an ionic compound minimize the distance between (1) charged ions while keeping (2)-charged ions away from each other
Hard
Melting point
Poor electrical conductor (solid)
Good electrical conductor (molten)
Brittle
BEHM
5 properties of ionic solids
Diamonds
Example of a covalent-network solid
Hard
Melting point, very high
Poor heat conductor
Poor electrical conductor
Brittle
5 properties of covalent-network solids
Molecular solid
A solid whose atoms are held together by van der Waals forces
Graphite
Example of molecular solid
Soft
Low melting point
Poor electrical conductor
Poor heat conductor
Brittle
SL PPB
5 properties of molecular solids
Phase change
Each state of matter can transform into either of the other two states
Heat of fusion
The energy required to change a solid at its melting point to a liquid
Heat of vaporization
The energy required to change a liquid at its boiling point to a gas
Heat of sublimation
The energy required to change a solid directly to a gas
Heat
The _____ added to the system at melting and boiling points goes into pulling the molecules farther apart from each other
Temperature
The _____ of the substance does not rise during a phase change
Phase diagrams
Display the state of a substance at various pressures and temperatures and the places where equilibria exist between phases
Triple point (T)
Critical point (C)
The liquid-vapor interface starts at the (1), at which all 3 states are in equilibrium, and ends at the (2), above which the liquid and vapor are indistinguishable from each other
Liquid crystals
Some substances don’t go directly from the solid state to the liquid state
They have some traits of both solids and liquids
Molecules have some degree of order
Nematic
Smectic
Cholesteryl
3 types of liquid crystals
Nematic liquid crystal
Type of liquid crystal
Molecules are only ordered in one dimension along the long axis
Smectic liquid crystals
Type of liquid crystal
Molecules are ordered into two dimensions along the long axis and in layers
Cholesteryl liquid crystals
Type of liquid crystal
Nematic-like crystals are layered at angles to each other
Solutions
Homogenous mixtures of two or more pure substances
Solute
Solvent
In a solution, the (1) is dispersed uniformly throughout the (2)
Natural tendency toward mixing
Intermolecular forces
The ability of substances to form solutions depends on
Spontaneous
Mixing of gases is a _____ process
Mixing
Entropy
(1) causes more randomness in the position of the molecules, increasing a thermodynamic quantity called (2)
Entropy
The formation of solutions is favored by the increase in _____ that accompanies mixing
Solute-solute
Solvent-solvent
Solvent-solute
Attractions formed when forming a solution
Solute-solute interactions
Attraction formed when forming a solution
Must be overcome to disperse these particles when making a solution
Solvent-solvent interactions
Attraction formed when forming a solution
Must be overcome to make room for the solute
Solvent-solute interactions
Attraction formed when forming a solution
Occur as the particles mix
Solubility
The maximum amount of solute that can dissolve in a given amount of solvent at a given temperature
Saturated solutions
Solutions that have the maximum amount of solute dissolved
Unsaturated solutions
Solutions that have any amount of solute less than the maximum amount dissolved in solution
Supersaturated solutions
The solvent of a solution holds more solute than is normally possible at that temperature
Unstable
Crystallization can usually be stimulated by adding “seed crystal” or scratching the side of the flask
Uncommon
Solute-solvent interactions
Pressure
Temperature
3 factors that affect solubility
Directly proportional
The relationship of solute-solvent interaction and solubility of a solute in that solvent
N2
O2
Ar
Kr
4 gases that only exhibit dispersion force
Directly proportional
The relationship of the size of a gas and its solubility in water
Polar
Nonpolar
(1) organic molecules dissolve in water better than (2) organic molecules
Hydrogen bonding
_____ increases solubility, since C—C and C—H bonds aren’t very polar
Miscible
Liquids that mix in all proportions are _____
Immiscible
Liquids that don’t mix in one another are _____
Solids
Liquids
The solubility of (1) and (2) aren’t appreciably affected by pressure
Henry’s Law
According to _____, the solubility of a gas is proportional to the partial pressure of the gas above the solution
Directly proportional
The relationship of most solids’ solubility and temperature
Unsaturation
Supersaturation
GRAPH Below the curve indicates (1) while above the curve indicates (2)
Inversely proportional
The relationship of gases’ solubility and the temperature
Mass percentage
Parts per million
Mole fraction
Molarity
Molality
5 units of concentration
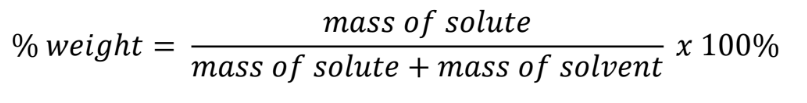
% weight = (mass of solute / mass of solution)(100)
w/w
Mass percentage formula
Unit
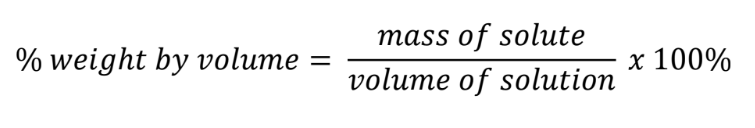
% weight by volume = (mass of solute / volume of solution)(100)
w/v
Percent weight by volume formula
Unit
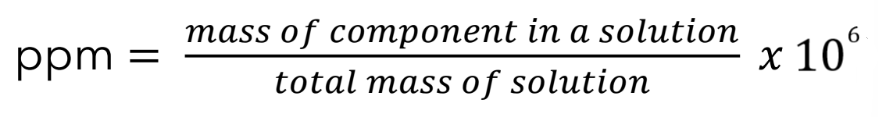
ppm = (mass of component in a solution / total mass of solution)(10^6)
Parts per million formula
mole fraction = moles of a component / moles of the solution
Mole fraction formula
Colligative properties
They depend only on the quantity, not the identity of the solute particles