Chem - Lecture 6: intermolecular Forces
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
What is Van der Waals (aka Dispersion Forces)?
Attraction of negatively charged electron clouds and positively charged nuclei of neighboring molecules.
What are Dipolar Forces?
Attraction between oppositely charged ends of neighboring molecules.
What are Hydrogen Bonds?
Bond between lone pair of electrons on N, O, or F atoms and a H atom.
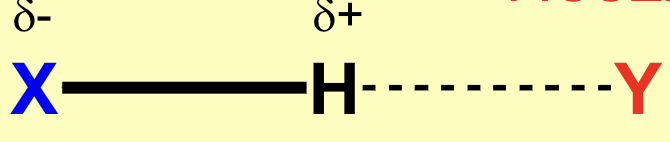
Which is the donor and which is the acceptor?
What does X and Y represent in hydrogen bonding?
X is donor and Y is acceptor.
Electronegativity of X and electron availability of Y.
What are the solvent properties of water?
Forms Ionic and Covalent compounds & its an environmental and biological solvent.
What are the thermal properties of water?
High heat capacity meaning:
1) Moderation of temp. of earth
2) Moderation of body temp.
3) Development of storms
What are ‘all’ the consequence of hydrogen bonding in water?
1) Solvent abilities
2) Density of water & ice
3) High heat capacity
4) High heat of vaporization
5) Surface tension