HAP22306 Theory week 3
0.0(0)
Card Sorting
1/110
There's no tags or description
Looks like no tags are added yet.
Last updated 10:15 AM on 5/2/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
111 Terms
1
New cards
16S rRNA for bacterial identification
Present in all bacteria, coding for an RNA component of the 30S subunit of the bacterial ribosome
2
New cards
ESKAPE
•Enterococcus faecium,
•Staphylococcus aureus, •
Klebsiella pneumoniae,
•Acinetobacter baumanii,
•Pseudomonas aeruginosa,
•Enterobacter species
•Staphylococcus aureus, •
Klebsiella pneumoniae,
•Acinetobacter baumanii,
•Pseudomonas aeruginosa,
•Enterobacter species
3
New cards
S.aureus characteristics
• S. aureus is found in the human commensal microbiota of the nasal mucosa in 20–40% of the general population.
•Resistance to methicillin a class of penicillin, emerged in hospital setting in 1961. Since then other more stable penicillin classes of antibiotics are used but there is resistance to all these
•Resistance to methicillin and, therefore, to most β-lactam antibiotics has occurred via horizontal gene transfer of staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that encodes the genes mecA or mecC.
•MRSA is often also resistant to multiple other antibiotic classes.
•Resistance to methicillin a class of penicillin, emerged in hospital setting in 1961. Since then other more stable penicillin classes of antibiotics are used but there is resistance to all these
•Resistance to methicillin and, therefore, to most β-lactam antibiotics has occurred via horizontal gene transfer of staphylococcal cassette chromosome mec (SCCmec), a mobile genetic element that encodes the genes mecA or mecC.
•MRSA is often also resistant to multiple other antibiotic classes.
4
New cards
MRSA Types and Symptoms
There are two ways a person can have MRSA: They can be a carrier or have an active infection. A carrier means that a person has no symptoms, but the MRSA bacteria are living in their nose or on their skin. This is also called colonization.An active infection means that the MRSA bacteria has entered the body through an opening (usually, a cut, scrape, or wound) and that person now has symptoms. There are also two types of MRSA infections, depending on where the MRSA was acquired. These two types are: Community-acquired MRSA (CA-MRSA) infections orHospital-acquired MRSA (HA-MRSA) infections
5
New cards
What is MSRA?
While there are many strains of the bacterium Staphylococcus aureus, or staph, methicillin-resistant Staphylococcus aureus (MRSA) is particularly notable because it is resistant to many standard antibiotics and may cause serious infections. Staph normally lives on the skin and sometimes in nasal passages. If an opening in the skin occurs, bacteria may enter the body and cause an infection. While MRSA infections are well-known to occur in people in care settings, such as hospitals, anyone can get MRSA.
6
New cards
Bacterial culturing
Growth conditions can vary (shaking or static, temperature, oxygen, time)Bacteria can be grown in different media, in liquid media or on solid media (plates)Media can be specific for some bacteria, to distinguish between species or genera
7
New cards
Bacterial growth curve (name the phases)
Lag phase;Exponential phase;Stationary phase;Death phase.
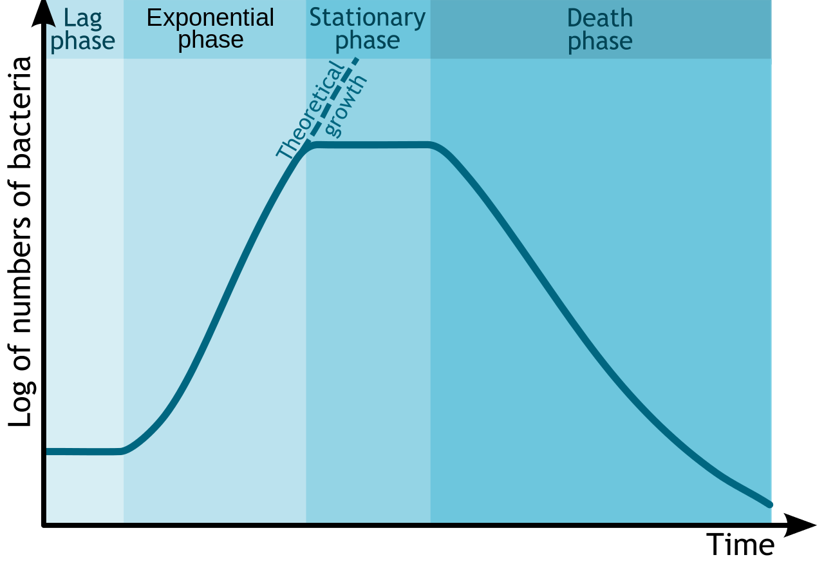
8
New cards
Colony formation
When growing on semi-solid agar plates in a petri dish, bacteria form colonies:
9
New cards
Colony morphology
Colonies grow in many different shapes, also depending on type of agar and type of bacteria: CircularPunctiformRhizoidFIlamentousIrregularSpindle
10
New cards
Clean streak
•A clean streak is performed to produce single colonies, for identification or further downstream processes Why do you want a single colony? To have bacteria with the same genetic make up •Place plate upside down in incubator overnight Why upside down? To prevent contamination and droplets falling down on the agar and disrupting the colony formation.
11
New cards
Mannitol salt agar (MSA):
High salt (NaCl) concentration in medium favors organisms that tolerate high salt concentrations, e.g. Staphylococcus, while being inhibitory for others. It is also a differential medium for mannitol fermenters, containing mannitol and the indicator phenol red. Staphylococcus aureus produce yellow colonies with yellow zones, whereas other Staphylococci produce small pink or red colonies with no color change to the medium. If an organism can ferment mannitol, an acidic byproduct is formed that will cause the phenol red in the agar to turn yellow.
12
New cards
API test
API test strips consists of microtubes (cupules) containing dehydrated substrates to detect the enzymatic activity or the assimilation fermentation of sugars by the inoculated organisms. During incubation, metabolism produces colour changes that are either spontaneous or revealed by the addition of reagents.
13
New cards
Catalase test
Test on the presence of catalase via H2O2
14
New cards
OD measurements, dilution and plating
The most common way to assess microbial growth in solution is the measurement of the optical density at 600 nm, or short OD600. The method is based on absorbance detection mode and basically determines which portion of light passes through a suspension of microorganisms.Calibration of OD with number of bacteria can vary per species and even strain – Why?
15
New cards
CFU vs OD graph
The optical density and colony forming units can be related, so that CFUs per milliliter can be estimated from OD 600 measurements, saving time and materials in future experiments. To do this, plot the colony forming units against the optical density on a linear scale for OD 600 readings less than or equal to 1.
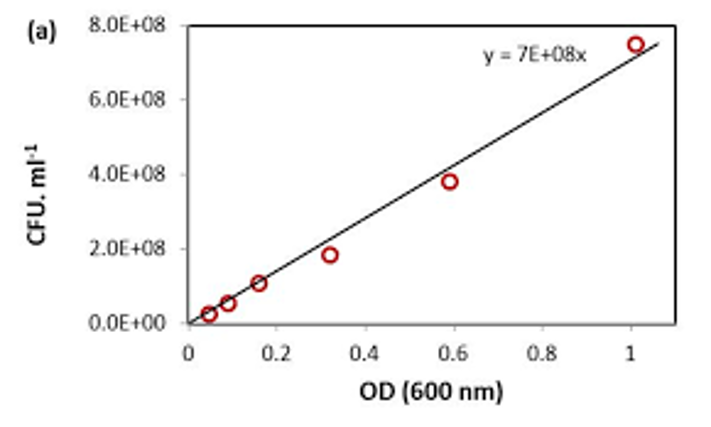
16
New cards
MIC assay
The minimum inhibitory concentration (MIC) is the lowest concentration of a chemical, usually an antibiotic, which prevents visible growth of a bacterial strain
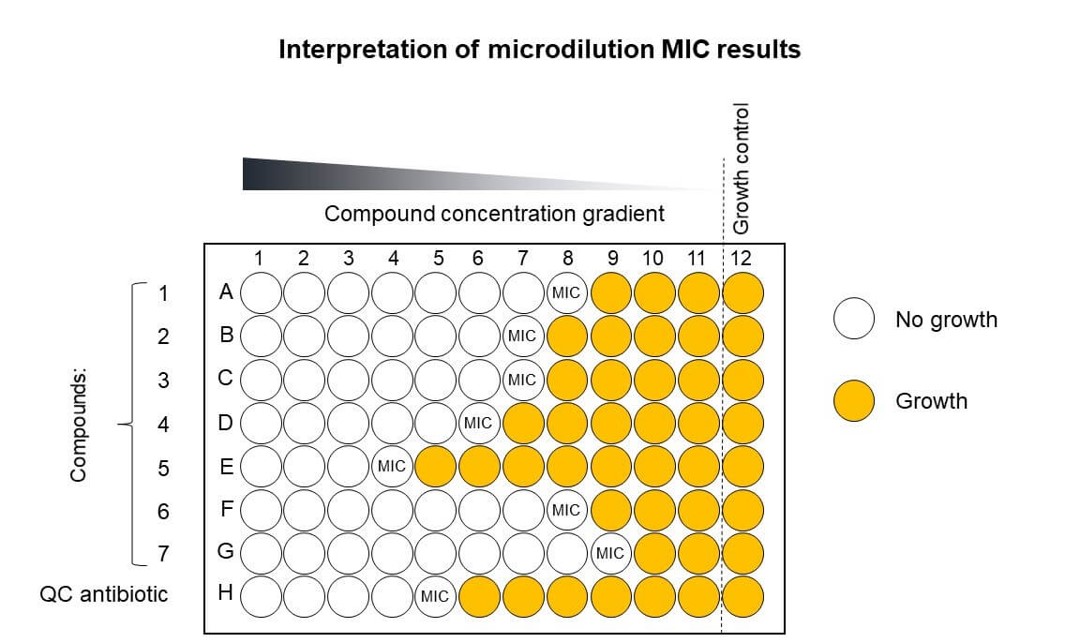
17
New cards
PCR
Polymerase chain reaction, a method to amplify/copy specific parts of the DNA, using specific components
18
New cards
Why perform a PCR?
* To multiply parts of DNA
\
For what?
* For visualization (a single DNA copy cannot be seen),
* to check: does a bacterium contain a certain gene?
* For cloning: a certain concentration of DNA is neededFor sequencing: to label your nucleotides
* To check: how highly is a gene expressed? (quantitative PCR)
\
For what?
* For visualization (a single DNA copy cannot be seen),
* to check: does a bacterium contain a certain gene?
* For cloning: a certain concentration of DNA is neededFor sequencing: to label your nucleotides
* To check: how highly is a gene expressed? (quantitative PCR)
19
New cards
How does a PCR work?
To amplify a segment of DNA using PCR, the sample is first heated so the DNA denatures, or separates into two pieces of single-stranded DNA.
Next, an enzyme called "Taq polymerase" synthesizes - builds - two new strands of DNA, using the original strands as templates. This process results in the duplication of the original DNA, with each of the new molecules containing one old and one new strand of DNA.
Then each of these strands can be used to create two new copies, and so on, and so on. The cycle of denaturing and synthesizing new DNA is repeated as many as 30 or 40 times, leading to more than one billion exact copies of the original DNA segment.
Next, an enzyme called "Taq polymerase" synthesizes - builds - two new strands of DNA, using the original strands as templates. This process results in the duplication of the original DNA, with each of the new molecules containing one old and one new strand of DNA.
Then each of these strands can be used to create two new copies, and so on, and so on. The cycle of denaturing and synthesizing new DNA is repeated as many as 30 or 40 times, leading to more than one billion exact copies of the original DNA segment.
20
New cards
What to think about when setting up a PCR reaction?
What to think about:
* Which gene/gene fragment do you want to amplify?
* For what purpose will you do the PCR?
* What will be your controls?
* Select the proper polymerase enzyme
* Design your primersTest your primers: optimize annealing temperature by doing a gradient PCR if needed
* Check product size by gel electrophoresis, and by sequencing to verify what you amplified
* Which gene/gene fragment do you want to amplify?
* For what purpose will you do the PCR?
* What will be your controls?
* Select the proper polymerase enzyme
* Design your primersTest your primers: optimize annealing temperature by doing a gradient PCR if needed
* Check product size by gel electrophoresis, and by sequencing to verify what you amplified
21
New cards
What is needed for a PCR reaction?
A polymerase buffer (optimized buffer, contains magnesium and loading dye)
* Polymerase (enzyme that builds a new DNA strand using a template)
* dNTP’s (nucleotides, the building blocks of the DNA strand)
* Primers (little pieces of DNA binding to the beginning and the end of the piece of DNA you want to amplify)
* Sterile water (to make the volume up to 20ul)
* Template DNA (the material of which you want to amplify a fragment)
* Final volume after adding 5 ul template is 25ul
* Every PCR reaction needs proper controls: a positive control to make sure the PCR reaction worked (machine was ok, everything was present in the mixture, etc), and a negative one to make sure all your reagents did not contain the target DNA.
* Polymerase (enzyme that builds a new DNA strand using a template)
* dNTP’s (nucleotides, the building blocks of the DNA strand)
* Primers (little pieces of DNA binding to the beginning and the end of the piece of DNA you want to amplify)
* Sterile water (to make the volume up to 20ul)
* Template DNA (the material of which you want to amplify a fragment)
* Final volume after adding 5 ul template is 25ul
* Every PCR reaction needs proper controls: a positive control to make sure the PCR reaction worked (machine was ok, everything was present in the mixture, etc), and a negative one to make sure all your reagents did not contain the target DNA.
22
New cards
Polymerase selection
There are different polymerases to chose from depending on your needs. Four basic properties of DNA polymerases can help you define the best enzyme for your particular research needs:
* 1. Thermal stability. A denaturation step at approximately 95°C in each PCR cycle separates the two strands of a DNA molecule. DNA polymerase must be robust enough to tolerate high-temperature cycles without compromising activity
* 2. Extension rate. This refers to the speed at which nucleotides are added, per second, per molecule of DNA polymerase, a factor determined by extension temperature, DNA template sequence and buffer composition. Current enzymes generate approximately 4 kb per minute!
* 3. Fidelity. Fidelity is an inherent DNA polymerase property defining the frequency of insertion of an incorrect nucleotide per kb of DNA.
* 4. Processivity. The probability that a polymerase will detach from DNA during extension. High processivity is important when amplifying long amplicons.
* Different configurations of these four variables have produced different classes of DNA polymerase, namely standard polymerases like Taq, as well as hot start, high fidelity and long-range polymerases
* 1. Thermal stability. A denaturation step at approximately 95°C in each PCR cycle separates the two strands of a DNA molecule. DNA polymerase must be robust enough to tolerate high-temperature cycles without compromising activity
* 2. Extension rate. This refers to the speed at which nucleotides are added, per second, per molecule of DNA polymerase, a factor determined by extension temperature, DNA template sequence and buffer composition. Current enzymes generate approximately 4 kb per minute!
* 3. Fidelity. Fidelity is an inherent DNA polymerase property defining the frequency of insertion of an incorrect nucleotide per kb of DNA.
* 4. Processivity. The probability that a polymerase will detach from DNA during extension. High processivity is important when amplifying long amplicons.
* Different configurations of these four variables have produced different classes of DNA polymerase, namely standard polymerases like Taq, as well as hot start, high fidelity and long-range polymerases
23
New cards
Polymerase buffer
* Based on which polymerase you have selected
* Buffers nowadays usually contain loading dye and magnesium
* Buffers nowadays usually contain loading dye and magnesium
24
New cards
dNTP’s
Add loose nucleotides as building blocksUse a standard concentration (0.5µl of 10nM), they are present in excess
25
New cards
Primer design
* Length typically 18-24 bases
* Annealing temperature 50 to 60 oC
* Start and end with 1-2 G/C pairs
* Product size
* Primer pairs should have a melting temperature ™ within 5°C of each other
* 40 -60% GC content
* No stable secondary structures like primer-dimers or hairpin formation
* Annealing temperature 50 to 60 oC
* Start and end with 1-2 G/C pairs
* Product size
* Primer pairs should have a melting temperature ™ within 5°C of each other
* 40 -60% GC content
* No stable secondary structures like primer-dimers or hairpin formation
26
New cards
Primer melting temperature (Tm)
Primer Melting Temperature (Tm) by definition is the temperature at which one half of the DNA duplex will dissociate to become single stranded and indicates the duplex stability
The primer melting temperature is the estimate of the DNA-DNA hybrid stability and critical in determining the annealing temperature. Too high Ta will produce insufficient primer-template hybridization resulting in low PCR product yield. Too low Ta may possibly lead to non-specific products caused by a high number of base pair mismatches,Tm and Ta should be close together to avoid nonspecific binding
The primer melting temperature is the estimate of the DNA-DNA hybrid stability and critical in determining the annealing temperature. Too high Ta will produce insufficient primer-template hybridization resulting in low PCR product yield. Too low Ta may possibly lead to non-specific products caused by a high number of base pair mismatches,Tm and Ta should be close together to avoid nonspecific binding
27
New cards
Undesirable primer interactions
Primer3’s thermodynamic models for predicting the stability of secondary structures. (A–C) Interactions between primers. (D) Hairpin structures. (E) Undesirable binding of primers to template sequence. Short white and black arrows represent forward and reverse primers, respectively. Gray arrows represent hybridization oligos. Long white and black arrows represent forward and reverse template. Curved oligos (including primers) indicate ANY interactions (A, B and D), and straight oligos indicate END (i.e. 3′-anchored) interactions (C and E).
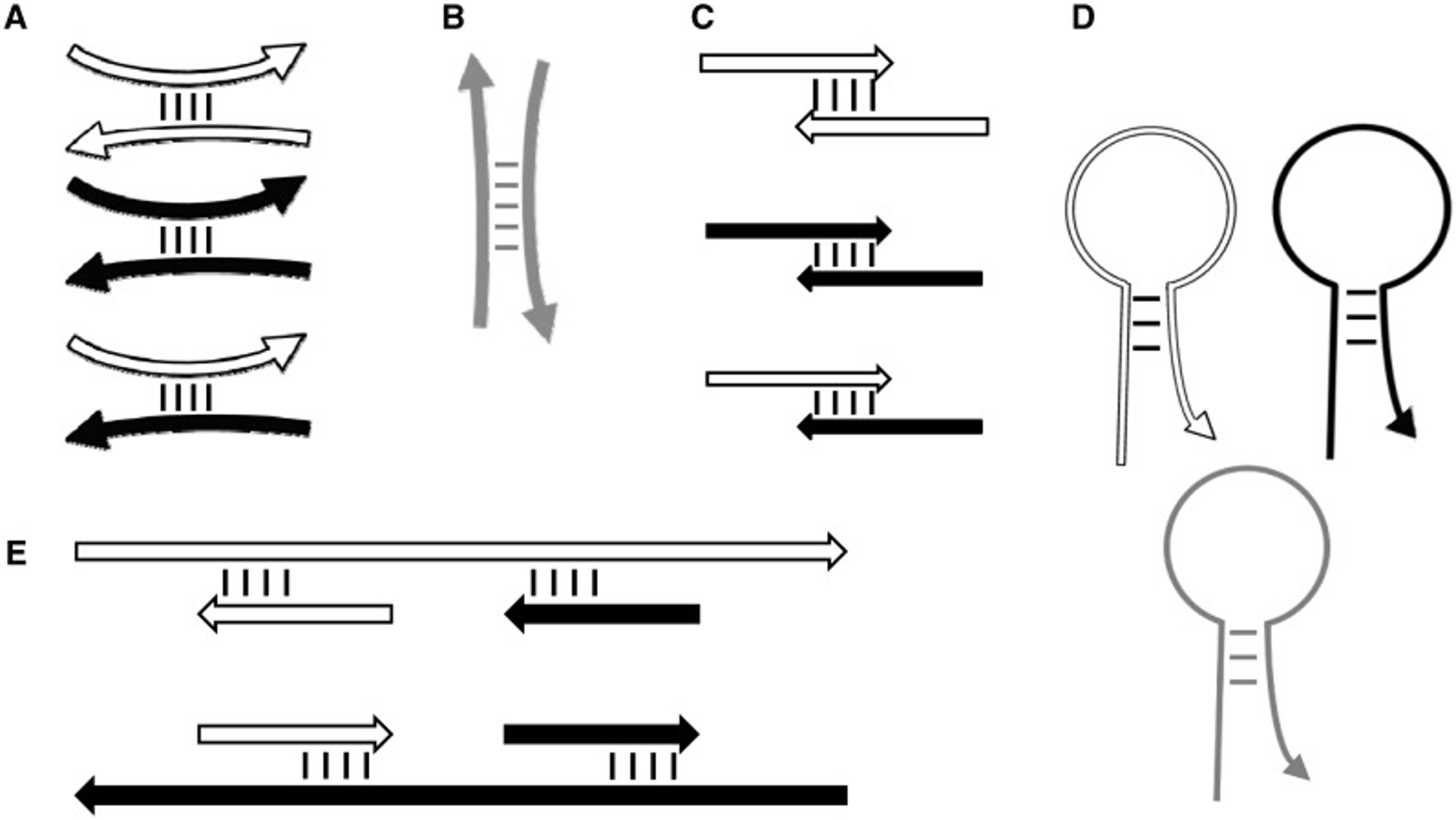
28
New cards
SYBR vs Taqman fluorescence
SYBR Green is a method based on intercalating nucleic acid staining dye while Taqman is a method based on hydrolysis probe.
29
New cards
What to do after a PCR?
* Visualize on a gel (is there a band, yes or no, how big is the band)
* Purify directly from PCR reaction (for downstream processing)
* Isolate from a gel (for downstream processing) •Analyse gene expression (quantitative PCR)
* Sequence your DNA
* Purify directly from PCR reaction (for downstream processing)
* Isolate from a gel (for downstream processing) •Analyse gene expression (quantitative PCR)
* Sequence your DNA
30
New cards
Principle of gel electrophoresis
* Load the samples into the wells
* Samples contain a loading dye, for visualization and to let it sink in the well
* Load a DNA ladder (molecular weight marker) •Apply a current
* DNA is negatively charged, so will move towards the positive pole (make sure you place your gel correct)
* Make sure to stop the current on time: the marker dye should not run off the gel
* Visualization by UV light: a dye (SYBRsafe) is added that binds dsDNA, which will light up under UV light
* Samples contain a loading dye, for visualization and to let it sink in the well
* Load a DNA ladder (molecular weight marker) •Apply a current
* DNA is negatively charged, so will move towards the positive pole (make sure you place your gel correct)
* Make sure to stop the current on time: the marker dye should not run off the gel
* Visualization by UV light: a dye (SYBRsafe) is added that binds dsDNA, which will light up under UV light
31
New cards
What can go wrong in gel electrophoresis?
* Run at too high voltage
* Pipet the sample into the well to vigorously
* Wait too long with turning on the current: DNA diffuses
* Loading too much sample
* Pipet the sample into the well to vigorously
* Wait too long with turning on the current: DNA diffuses
* Loading too much sample
32
New cards
Different methods to sequence DNA
* Sanger
* Next generation (MiSeq, HiSeq)
* Pyrosequencing
* MinION sequencing
* Next generation (MiSeq, HiSeq)
* Pyrosequencing
* MinION sequencing
33
New cards
Sanger sequencing
A laser excites the dye labeled DNA fragments as they pass through a tiny window at the end of the capillary. The excited dye emits a light at a characteristic wavelength that is detected by a light sensor. Software can then interpret the detected signal and translate it into a base call.
34
New cards
Advantages and limitations sanger sequencing
Advantages:
* Rapid, robust technique
* Accurate
* Cost effective
Limitations:
* Only suitable for short pieces of DNA (app 300 to 1000 bp) Can be used for:
* Targeting smaller genomic regions in a larger number of samples, sequencing of variable regions, validating results from next-generation sequencing (NGS) studies, verifying plasmid sequences, inserts, mutations, HLA typing
* Rapid, robust technique
* Accurate
* Cost effective
Limitations:
* Only suitable for short pieces of DNA (app 300 to 1000 bp) Can be used for:
* Targeting smaller genomic regions in a larger number of samples, sequencing of variable regions, validating results from next-generation sequencing (NGS) studies, verifying plasmid sequences, inserts, mutations, HLA typing
35
New cards
Advantages next generation sequencing
In principle: concepts between NGS and Sanger are similar.
* High sensitivity to detect low-frequency variants
* Fast turnaround time for high sample volume
* Ability to sequence hundreds to thousands of genes simultaneously
* Capacity with sample multiplexing
* High sensitivity to detect low-frequency variants
* Fast turnaround time for high sample volume
* Ability to sequence hundreds to thousands of genes simultaneously
* Capacity with sample multiplexing
36
New cards
Advantages minION sequencing
Advantages:
* Powerful
* Portable: small device, to carry with you into the field for example
* Real-time
* Unrestricted read length (from short to >4Mb)
Disadvantage:
* More prone to errors than other methods
* Powerful
* Portable: small device, to carry with you into the field for example
* Real-time
* Unrestricted read length (from short to >4Mb)
Disadvantage:
* More prone to errors than other methods
37
New cards
How do you recognize S.aureus on a MSA plate?
\
\
S.aureus is visible as yellow colonies with yellow zones.
38
New cards
Why do you grow plates upside down in an incubator?
\
\
\
\
To prevent droplets from condensation falling on the agar with the bacteria. This can disturb the bacteria and colour formation. And to prevent contamination.
39
New cards
What is the difference between using a biosafety hood and a flame? Think about protecting your work and yourself.
\
\
Both share a common objective – the reduction of contamination in the microbiological laboratory.
However, in term of protecting your work, a flame creates a sterile environment while a biosafety hood does not.
In terms of protecting yourself, a hood prevents contamination of bacteria with yourself (HEPA filter), so you have a protection barrier, while a flame does not (think about spatting up of fluids/bacteria).
However, in term of protecting your work, a flame creates a sterile environment while a biosafety hood does not.
In terms of protecting yourself, a hood prevents contamination of bacteria with yourself (HEPA filter), so you have a protection barrier, while a flame does not (think about spatting up of fluids/bacteria).
40
New cards
Which control should we take along in this experiment?
\
\
A negative control, so culturing growth medium without S.aureus to ensure that there is no other bacterial growth.
The positive control is your sample.
The positive control is your sample.
41
New cards
We will use a loose cap tube for the liquid culture, why?
Loose fitting caps or cotton plug permits free passage of air over the edge of the tubes or flasks and prevent the establishment of semi-anaerobic conditions in the culture.
42
New cards
When would you want to use the plate method to calculate the number of bacteria
\
\
You would want to use the plate method to calculate the number of viable bacteria in a sample.
This method is particularly useful when you need to determine the number of bacteria that are capable of growing and reproducing under specific conditions, such as in a particular environment, in response to a particular treatment or antibiotic, or in the presence of other microorganisms. It is also commonly used in microbiology research and testing, as it provides a quantitative measure of the number of bacteria present in a sample.
This method is particularly useful when you need to determine the number of bacteria that are capable of growing and reproducing under specific conditions, such as in a particular environment, in response to a particular treatment or antibiotic, or in the presence of other microorganisms. It is also commonly used in microbiology research and testing, as it provides a quantitative measure of the number of bacteria present in a sample.
43
New cards
When would you want to measure OD600 to calculate the number of bacteria
You would want to measure OD600 to monitor the growth of bacterial cultures over time. So it is handy for estimating the concentration of bacterial cells in a liquid culture. However, it does not distinguish between viable and non-viable cells, so it may not be suitable for all applications where precise quantification of bacterial viability is required.
44
New cards
What are the 3 phases in PCR?
\
Denaturing, enabling and extension.
Denaturing, enabling and extension.
45
New cards
What does the polymerase do in a PCR reaction?
\
\
Polymerase enzyme synthesizes long chains of nucleic acids
46
New cards
Why do we have a longer denaturing step in this PCR reaction?
\
\
To lysis bacteria. And free the DNA.
47
New cards
What happens when you put the lid on the tray wrong?
\
\
The DNA will run upwards.
48
New cards
When you have a very small DNA fragment, would you use a 0.8 or a 2% agarose gel?
\
\
2%, to let it run slower through the gel; have more resistance.
49
New cards
What happens when you leave the gel for too long?
\
\
The DNA will run off the gel.
50
New cards
Why do you add a loading dye to your sample?
\
\
To see the migration, glycerol will make the product sink in the well, and to visualize DNA.
51
New cards
What can cause smears on the gel?
Loading too much DNA
52
New cards
* What is the importance of sterile techniques in microbial cell culture?
\
\
* Sterile techniques are important in microbial cell culture to prevent accidental contamination of pure cultures with other organisms.
\
\
53
New cards
* Why is it important to protect the operator during microbial cell culture?
\
\
* It is important to protect the operator during microbial cell culture, especially from potentially harmful organisms.
\
\
54
New cards
* What safety conditions should be adopted during microbial cell culture?
\
\
* Aseptic techniques and safety conditions described for animal cell culture should be adopted at all times.
\
\
55
New cards
* How should instruments be sterilized in microbial cell culture?
\
\
* Instruments used during the culturing procedures should be sterilized before and after use by heating in a Bunsen burner flame.
\
\
56
New cards
* Why should work areas be decontaminated after use in microbial cell culture?
\
\
* Work areas should be decontaminated after use in microbial cell culture to prevent airborne bacteria from spreading rapidly.
\
\
57
New cards
How should materials used in microbial cell culture be disposed of?
Materials used in microbial cell culture should be disposed of appropriately, for instance, by autoclaving all plastics and tissue culture waste before disposal.
58
New cards
* What are the conditions required for bacterial growth?
\
\
* Bacterial growth requires much simpler conditions than those for animal cells.
\
\
59
New cards
* What determines the composition of the medium used for bacterial culture?
\
\
* The composition of the medium used for bacterial culture may vary and is largely determined by the nutritional classification of the organisms being cultured.
\
\
60
New cards
* What are the two main categories of nutritional classification for bacteria?
\
\
* The two main categories of nutritional classification for bacteria are autotrophs and heterotrophs.
\
\
61
New cards
* What are autotrophs and heterotrophs in bacterial culture?
\
\
* Autotrophs are self-feeding organisms that synthesise their own food in the form of sugars, while heterotrophs are non-self-feeding organisms that derive chemical energy by breaking down organic molecules consumed.
\
\
62
New cards
* What are the subgroups of autotrophs and heterotrophs?
\
\
* The subgroups of autotrophs and heterotrophs include chemo- or photoautotrophs or -heterotrophs.
\
\
63
New cards
How do chemo- and photoautotrophs differ in their sources of energy?
Chemo- and photoautotrophs differ in their sources of energy, with chemoautotrophs utilising inorganic substances and photoautotrophs using light.
64
New cards
* What are the two categories of medium used to culture bacteria?
\
\
* The two categories of medium used to culture bacteria are complex and defined.
\
\
65
New cards
* What does complex medium usually consist of?
\
\
* Complex medium usually consists of natural substances, including meat and yeast extract, and is less well defined.
\
\
66
New cards
* What are the advantages of using complex medium for bacterial culture?
\
\
* The advantages of using complex medium for bacterial culture include its richness in nutrients and suitability for culturing fastidious organisms that require a mixture of nutrients for growth.
\
\
67
New cards
* What are defined media in bacterial culture?
\
\
* Defined media in bacterial culture are relatively simple and are made up of known components put together in the required amounts.
\
\
68
New cards
* How are defined media designed?
\
\
* Defined media are designed to the specific needs of the bacterial species to be cultivated.
\
\
69
New cards
How can certain species of bacteria be selected or eliminated using defined media?
Certain species of bacteria can be selected or eliminated using defined media by taking advantage of their distinguishing nutritional requirements, such as by including bile salts to inhibit the growth of most other Gram-positive and Gram-negative bacteria when selective cultivation of enteric bacteria is required.
70
New cards
* What are the two types of media used to culture bacteria?
\
\
* The two types of media used to culture bacteria are liquid and solid.
\
\
71
New cards
* How are liquid cultures agitated?
\
\
* Liquid cultures are agitated continuously on a shaker that rotates in an orbital manner.
\
\
72
New cards
* What is the recommended volume of medium in a flask for aerobic bacteria?
\
\
* The recommended volume of medium in a flask for aerobic bacteria should not exceed more than 20% of the total volume of the flask.
\
\
73
New cards
* What are fermenters/bioreactors and what are they equipped with?
\
\
* Fermenters/bioreactors are large-scale culture devices equipped with stirring devices for improved mixing and gas exchange, as well as probes that monitor changes in pH, oxygen concentration, and temperature. They are surrounded by a water jacket with fast-flowing cold water to reduce the heat generated during fermentation. Outlets are also included to release CO2 and other gases produced by cell metabolism.
\
\
74
New cards
* What is the purpose of solid agar media?
\
\
* The purpose of solid agar media is to separate mixed cultures and form the basis for isolation of pure cultures of bacteria.
\
\
75
New cards
* What is the difference between batch and continuous cultures?
\
\
* Batch cultures entail inoculating an aliquot of cells into a sterile flask containing a finite amount of medium, while continuous cultures refresh the medium regularly to replace that spent by the cells. The objective of the latter is to maintain the cells in the exponential growth phase by enabling nutrients, biomass, and waste products to be controlled through varying the dilution rate of the cultures.
\
\
76
New cards
* What are the advantages of continuous cultures over batch cultures?
\
\
* Continuous cultures offer certain advantages over batch cultures in that they facilitate growth under steady-state conditions in which there is tight coupling between cell division and biosynthesis. As a result, the physiological status of the cultures is more clearly defined, with very little variation in the cellular composition of the cells during the growth cycle.
\
\
77
New cards
What is the main concern with the open system of continuous cultures?
The main concern with the open system of continuous cultures is the high risk of contamination associated with the dilution of the cultures. However, applying strict aseptic techniques during feeding or harvesting cells may help to reduce the risk of such contamination. The whole system can also be automated to minimize direct contact with the operator or outside environment, thereby reducing the risk of contamination.
78
New cards
Electrophoresis
* Electrophoresis in agarose or polyacrylamide gels is the usual way to separate DNA molecules according to size.
* Large fragments of DNA may also be separated by a modification of electrophoresis termed pulsed-field gel electrophoresis (PFGE).
* Electrophoresis can be used analytically or preparatively and can be qualitative or quantitative.
* Intercolation dyes (e.g. SYBR® Green or ethidium bromide) can be used to stain DNA and exhibit strong fluorescence when illuminated with ultraviolet light.
\
* Large fragments of DNA may also be separated by a modification of electrophoresis termed pulsed-field gel electrophoresis (PFGE).
* Electrophoresis can be used analytically or preparatively and can be qualitative or quantitative.
* Intercolation dyes (e.g. SYBR® Green or ethidium bromide) can be used to stain DNA and exhibit strong fluorescence when illuminated with ultraviolet light.
\
79
New cards
Pulsed-field gel electrophoresis (PFGE)
\
\
Pulsed-field gel electrophoresis (PFGE) is a modification of electrophoresis used to separate large fragments of DNA such as chromosomes
80
New cards
Minigels
* Minigels are particularly convenient for checking purity and intactness of DNA preparation or to assess enzymatic reactions.
* Minigels use small sample volumes and give results quickly.
\
* Minigels use small sample volumes and give results quickly.
\
81
New cards
Agarose gels
* Agarose gels can be used to separate molecules larger than about 100 bp, while polyacrylamide gels are preferred for higher resolution or shorter DNA molecules.
\
\
82
New cards
Preparative electrophoresis
Preparative electrophoresis involves physically removing the gel containing the desired DNA fragment, and the DNA can be recovered from the gel fragment in various ways, including electroelution.
* Preparative electrophoresis involves physically removing the gel containing the desired DNA fragment.
* DNA may be recovered from the gel fragment in various ways, including electroelution.
* Preparative electrophoresis involves physically removing the gel containing the desired DNA fragment.
* DNA may be recovered from the gel fragment in various ways, including electroelution.
83
New cards
Intercolation dyes
* Intercolation dyes (e.g. SYBR® Green or ethidium bromide) can be used to stain DNA by inserting between stacked base pairs.
* Intercolation dyes exhibit strong fluorescence when illuminated with ultraviolet light.
* Intercolation dyes exhibit strong fluorescence when illuminated with ultraviolet light.
84
New cards
* What holds the two anti-parallel strands of DNA together?
\
\
* The weak forces of hydrogen bonding between complementary bases and hydrophobic interactions between adjacent, stacked base pairs.
\
\
85
New cards
* What is denaturation?
\
\
* The separation of the two strands of DNA into single strands due to high temperature.
\
\
86
New cards
* What is the hyperchromic effect?
\
\
* The increase in the absorbance of light at 260 nm as the DNA becomes denatured.
\
\
87
New cards
* What is the melting temperature (Tm)?
\
\
* The temperature at which 50% of the DNA is melted.
\
\
88
New cards
* How is Tm related to the (C + G) content of DNA?
\
\
* Tm is highest for DNA molecules that contain the highest proportion of cytosine and guanine.
\
\
89
New cards
* What is renaturation?
\
\
* The process by which the separated strands of DNA reassociate.
\
\
90
New cards
* What is hybridization?
\
\
* The pairing of complementary strands of RNA and DNA.
\
\
91
New cards
What are oligonucleotides?
Small single-stranded fragments of DNA up to 40 bases in length that can hybridize to a denatured sample of DNA.
92
New cards
What is uracil N-glycosylase (UNG) used for?
UNG is an enzyme that degrades any PCR amplicons with incorporated uracil, rendering them useless as templates.
93
New cards
What is a hotstart in PCR?
A hotstart is a method used in PCR where the reaction mixture is physically separated from the template or enzyme to avoid any mispriming, achieved by means of a heat-labile chemical moiety or antibody binding to the DNA polymerase.
94
New cards
Sonication
This method is ideal for a suspension of cultured cells or microbial cells. A sonicator probe is lowered into the suspension of cells and high frequency sound waves (
95
New cards
Repeated Freeze–Thaw
A more gentle and readily available method for lysis of bacterial cells is to subject the aqueous cell suspension to repeated freezing and thawing conditions. Resuspended cells are frozen (either at −20 °C, −80 °C or in liquid nitrogen) and then thawed in warm water.
96
New cards
Blenders
These are commercially available, although a typical domestic kitchen blender will suffi ce. This method is ideal for disrupting mammalian or plant tissue by shear force. Tissue is cut into small pieces and blended, in the presence of buffer, for about 1 min to disrupt the tissue.
97
New cards
Grinding With Abrasives
Grinding with a pestle in a mortar, in the presence of sand or alumina and a small amount of buffer, is a useful method for disrupting bacterial or plant cells; cell walls are physically ripped off by the abrasive
98
New cards
Presses
The use of homogenisers such as a French press, or the Manton–Gaulin press, which is a larger-scale version, is an excellent means for disrupting microbial cells.
99
New cards
Enzymatic Methods
The enzyme lysozyme, isolated from hen egg whites, cleaves peptidoglycan. The peptidoglycan cell wall can therefore be removed from Gram-positive bacteria (see Figure 5.2 ) by treatment with lysozyme, and if carried out in a suitable buffer, the cell membrane will rupture, owing to the osmotic effect of the suspending buffer
100
New cards
* What is the starting material for protein purification?
\
\
* The starting material for protein purification is the initial extract obtained by disrupting cells or tissue to release protein content into an appropriate buffer, unless the protein is secreted.
\
\