Chemistry 3.8- Aldehydes and Ketones
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Outline the general mechanism for the reduction of an aldehyde- what kind of mechanism is this?
Nucleophilic addition, leaves a primary hydroxy group
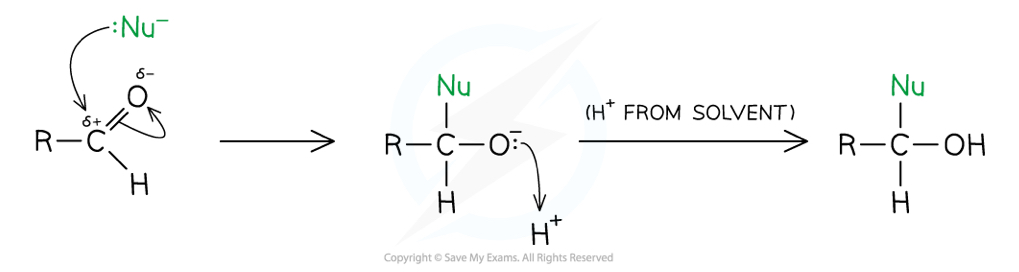
Outline the general mechanism for the reduction of an ketone- what kind of mechanism is this?
Nucleophilic addition, leaves a secondary hydroxyl group

What reactant is commonly used for the reduction of aldehydes and ketones to primary and secondary alcohols?
Aqueous NaBH₄ (sodium borohydride or sodium tetrahydridoborate), which provides hydride ions (:H⁻), a nucleophile
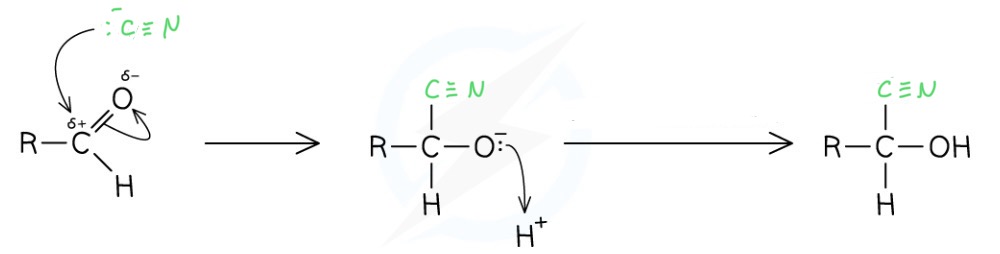
Outline the reaction and mechanism for the reduction of an aldehyde by cyanide ions
React with KCN followed by a dilute acid
Produces a hydroxynitrile
The chain length is increased by one
This is a nucleophilic addition reaction
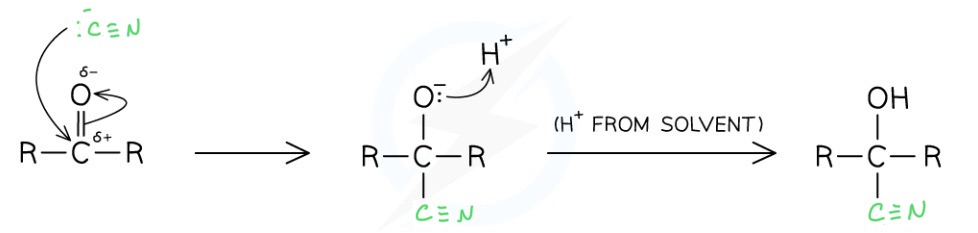
Outline the reaction and mechanism for the reduction of a ketone by cyanide ions
React with KCN followed by a dilute acid
Produces a hydroxynitrile
The chain becomes more branched because a carbon has been added
This is a nucleophilic addition reaction
Why can sodium borohydride reduce aldehydes and ketones but not alkenes?
The nucleophilic hydride ions (:H⁻) are attracted to the delta positive carbon in the polar C=O bond found in aldehydes and ketones, so they can undergo nucleophilic addition
However hydride ions are repelled by the electron density in a C=C bond, which is not polar, so they can’t undergo nucleophilic addition
What is the general equation for the reduction of an aldehyde, using [H] as the reducing agent?
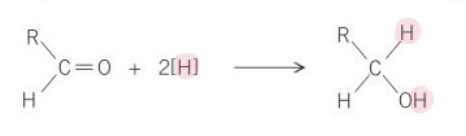
What is the general equation for the reduction of a ketone, using [H] as the reducing agent?
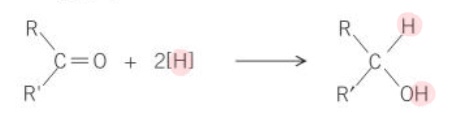
What is the hazard of the reduction of aldehydes and ketones by potassium cyanide?
Why do we use it anyways, instead of HCN?
Cyanide is highly toxic
We still use it because potassium cyanide dissociates into cyanide ions more easily
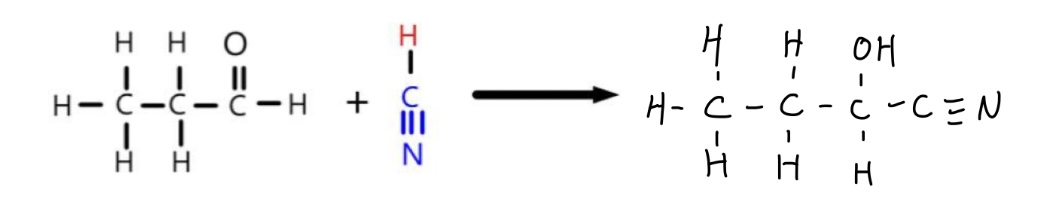
What is the overall equation for the reduction of propanal by cyanide ions? What is the product of this reaction?
KCN and a dilute acid is commonly used to provide cyanide ions and hydrogen ions, but this can be simplified in equations to writing HCN
This produces 2-hydroxybutanenitrile, as a carbon is added onto the chain
Why do nucleophilic addition reaction products of aldehydes and ketones with cyanide ions have no effect on plane polarised light?
The cyanide ion nucleophile can attack the carbonyl carbon from either side of the plane
This produces a pair of enantiomers (opposite optical isomers)
There is no preference for the reaction so the two enantiomers will be produced with equal probability
This produces a racemic (1:1) mixture
A pair of enantiomers will rotate the plane of plane-polarised light the same amount in opposite directions
The effects are cancelled out so there is no net rotation and the mixture has no effect on plane polarised light
Why do nucleophilic addition reaction products of unsymmetrical ketones with hydride ions have no effect on plane polarised light?
The hydride ion nucleophile can attack the carbonyl carbon from either side of the plane
This produces a pair of enantiomers (opposite optical isomers)
There is no preference for the reaction so the two enantiomers will be produced with equal probability
This produces a racemic (1:1) mixture
A pair of enantiomers will rotate the plane of plane-polarised light the same amount in opposite directions
The effects are cancelled out so there is no net rotation and the mixture has no effect on plane polarised light
Why do the nucleophilic addition reactions of aldehydes with hydride ions have no effect on plane polarised light?
The carbonyl carbon already has a hydrogen attached as part of the aldehyde group
So when another is added, there won’t be 4 different groups around the carbon
This means there is no optical centre
It won’t rotate the plane of plane polarised light