8. Invertebrate Early Development II: The Drosophila body plan or Segmentation
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
What is segmentation in biology, and is it a conserved feature?
Segmentation is the division of an animal's body plan into a series of repetitive segments.
It is an ancient and highly conserved way of building bodies, observed in diverse phyla including annelids (like leeches), arthropods (like insects), and vertebrates (e.g., vertebral column, somites).
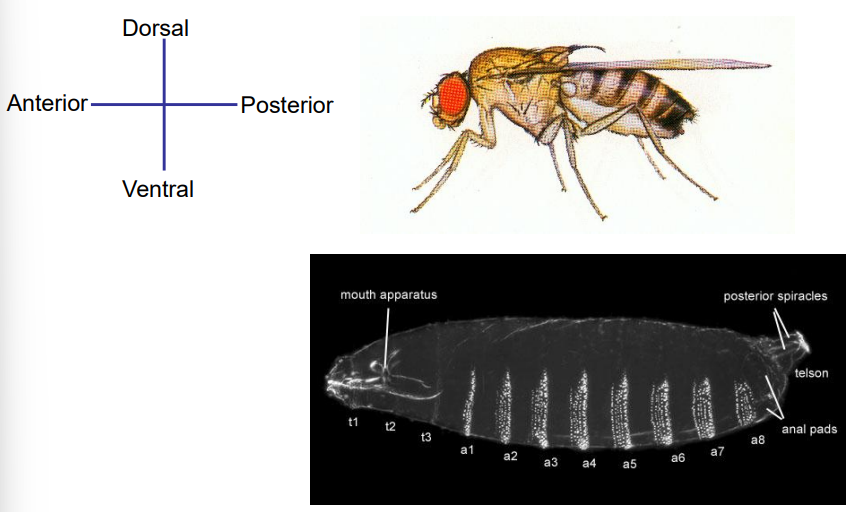
How are insects, like vertebrates, typically organized in terms of body axes?
Insects, like vertebrates, are bilaterally symmetrical.
They have two distinct and largely independent primary body axes: the Anterior-Posterior (A-P) axis, running from head to tail, and the Dorsal-Ventral (D-V) axis, running from back to belly.
Patterning Set-up
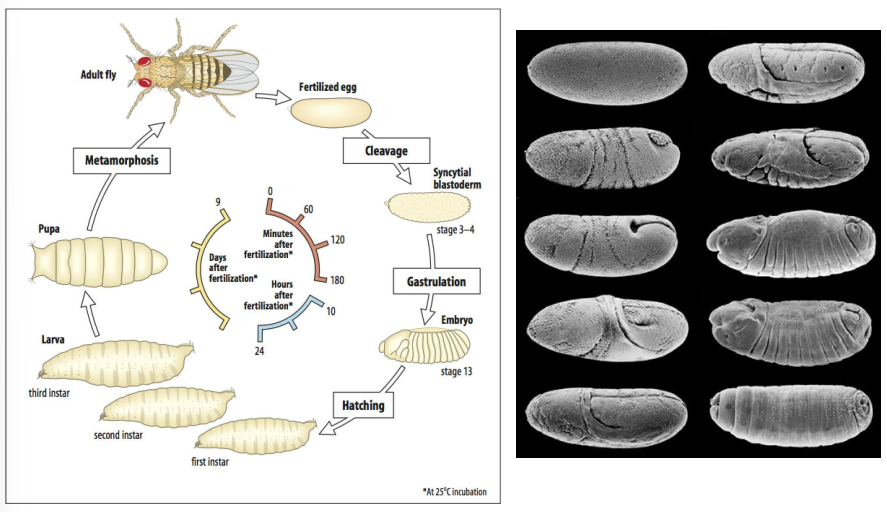
When is body patterning initiated in Drosophila development?
What is this due to?
What do these factors establish?
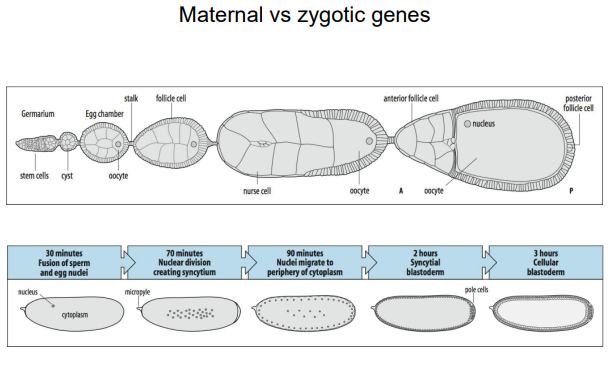
In Drosophila, the fundamental body patterning is already set up in the egg, even before fertilization.
This is due to the maternal contribution of RNAs and proteins that are asymmetrically localized within the oocyte during oogenesis.
These maternal factors establish the initial A-P and D-V axes.
The Heidelberg Screen
When was the Heidelberg screen published and when did the it win the nobel prize?
What was the Heidelberg screen?
What genes did it distinguish between?
The Heidelberg Screen, published in 1980, won the Nobel Prize in 1995. It was conducted by Nüsslein-Volhard and Wieschaus.
The heidelberg screen was a large-scale mutagenesis screen that identified almost all of the key genes involved in setting up the body axes and segment pattern in Drosophila.
It distinguished between maternal-effect genes (whose products are supplied by the mother to the egg) and zygotic genes (transcribed from the embryo's own genome after fertilization).
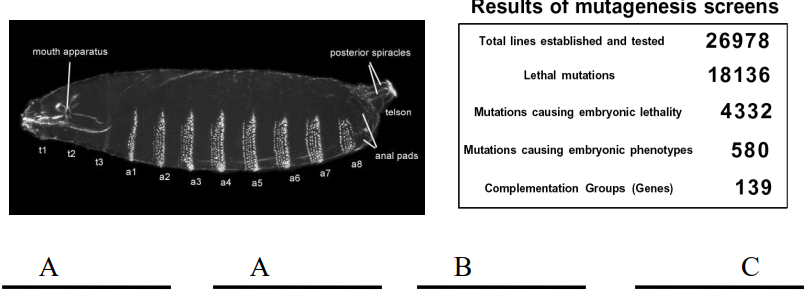
The Heidelberg Screen
What types of mutations were the primary focus of the Heidelberg screen, and what key observable phenotype did they look for?
What percentage of the lethal mutations were embryonic lethal mutations?
Why did the Heidelberg screen "largely exclude" viable mutants?
What are "maternal effect phenotypes," and why did the Heidelberg screen "miss" genes responsible for them?
What kind of genes was the Heidelberg screen primarily designed to identify, regarding their expression in the embryo?
How were "complementation groups" identified in the Heidelberg screen through crossing mutant lines?
Approximately how many unique genes were found to be essential for the Drosophila larval body plan?
The Heidelberg screen primarily focused on embryonic lethal mutations that caused visible defects in the larval cuticle pattern.
Only around 25%.
It excluded viable mutants because its goal was to identify genes with profound, early effects on the body plan that led to embryonic death. Mutations not causing lethality or obvious cuticle defects were overlooked.
Maternal effect phenotypes occur when a mutation in the mother's genome (specifically when the mother is homozygous for the mutation) affects the offspring's development, regardless of the offspring's own genotype. The Heidelberg screen missed these because it looked for zygotic mutations (mutations expressed by the embryo itself), and a heterozygous mother (as used in the screen) would supply enough functional maternal product to mask any such defect.
The screen was designed to identify genes with zygotic mutations, meaning those whose products are produced by the embryo's own genome during development.
Complementation tests classified mutations: if 2 mutant lines crossed to produce wild-type offspring, their mutations complemented (different genes) - this is because each parent could supply a functional copy of the gene mutated in the other. If they produced mutant offspring, they failed to complement (same gene/alleles).
This analysis identified approximately 140 unique genes (complementation groups) vital for the Drosophila larval body plan. These genes were then classified into distinct phenotypic classes, such as maternal effect, gap, pair-rule, segment polarity, and homeotic genes, which illuminated the hierarchical control of pattern formation.
What is the difference between maternal-effect genes and zygotic genes in Drosophila development?
Maternal-effect genes are transcribed in the mother's ovarian nurse cells, and their mRNA or protein products are deposited into the developing oocyte. Mutations in these genes in the mother lead to defects in the embryo, regardless of the embryo's own genotype. They establish the initial polarity of the egg.
Zygotic genes are transcribed from the embryo's own genome after fertilization. Their expression is often regulated by maternal gene products and earlier-acting zygotic genes. Mutations in zygotic genes affect the embryo's development based on its own genotype.
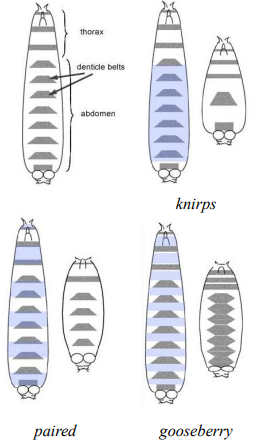
What are the 3 main classes of zygotic patterning genes identified by the Heidelberg Screen based on their mutant cuticle phenotypes?
Name some examples for each and their mutant cuticle phenotype.
The screen identified several classes of zygotic patterning genes:
1. Gap genes (e.g., knirps, Krüppel, hunchback): Mutants lack large contiguous blocks of segments.
2. Pair-rule genes (e.g., paired, even-skipped, fushi tarazu): Mutants lack portions of every other segment, resulting in a pattern of alternating missing parasegments (often 7 stripes of expression).
3. Segment polarity genes (e.g., gooseberry, wingless, hedgehog): Mutants show defects in patterning within each segment, often affecting the anterior-posterior polarity or structures within every segment.
Describe the hierarchical cascade of gene activity in Drosophila segmentation. Describing what they each establish, and if they are activated by a specific product.
Maternal genes
Gap genes
Pair-rule genes
Segment polarity genes
The segmentation gene cascade proceeds as follows:
Maternal genes: Establish broad anterior-posterior (A-P) and dorsal-ventral (D-V) gradients of morphogens (e.g., Bicoid protein in the anterior). They lay down the initial coordinates.
Gap genes: Activated by maternal gene products. They are expressed in broad, overlapping domains along the A-P axis, dividing the embryo into several large regions.
Pair-rule genes: Activated by combinations of maternal and gap gene proteins. They are typically expressed in seven transverse stripes, establishing parasegmental boundaries.
Segment polarity genes: Activated by pair-rule gene products. They are expressed in a strip of cells within each parasegment and refine the segment boundaries and establish polarity within each segment.
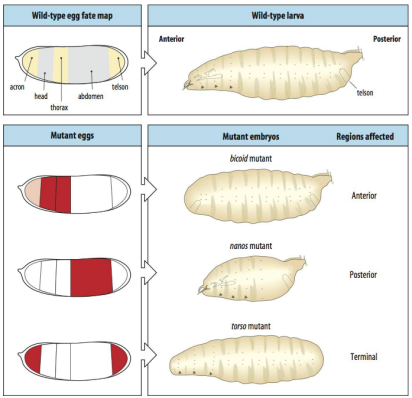
Maternal genes
What would happen if you had a bicoid mutant?
What would happen if you had a nanos mutant?
What would happen if you had a torso mutant?
In a bicoid mutant the ANTERIOR regions are affected (the head, thorax).
In a nanos mutant the POSTERIOR regions are affected (the abdomen).
In a torso mutant the TERMINAL (end) regions are affected (the acron and telson).
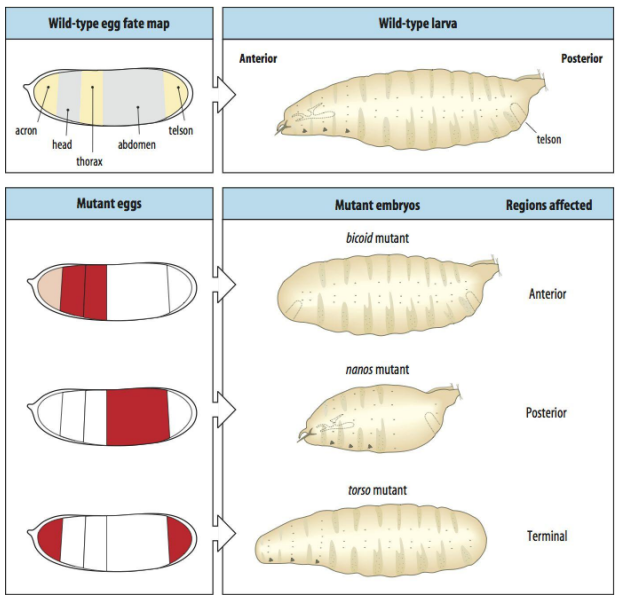
Bicoid
What is Bicoid?
What is bicoid mRNA deposited by and where?
After fertilisation of the embryo, what happens to the bicoid mRNA?
What does the bicoid protein do?
What does a bicoid concentration gradient do?
How does it function as a maternal gene to determine anterior structures in Drosophila?
Bicoid (bcd) is a maternal-effect gene.
Bicoid mRNA is deposited by nurse cells into the anterior pole of the developing oocyte during oogenesis.
After fertilization, this mRNA is translated.
The bicoid protein, a DNA-binding transcriptional activator (a homeodomain transcription factor), forms a concentration gradient, highest at the anterior and decreasing towards the posterior.
This gradient determines anterior fates; high Bicoid levels specify head structures, while lower levels specify thoracic structures. It is a morphogen.
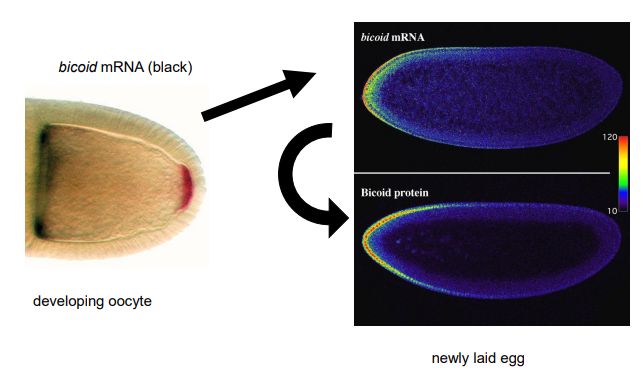
How do classic experiments demonstrate that Bicoid acts as a morphogen in Drosophila? What happens in each?
Normal development
Bicoid mutant egg
Rescue experiment
Ectopic expression
Normal development: Wild-type egg develops into a normal larva.
Loss of function (bicoid mutant egg): Results in a larva lacking anterior structures (head and thorax), often with posterior structures (telson) duplicated at the anterior end.
Rescue experiment: Injecting cytoplasm from the anterior of a wild-type egg into the anterior of a bicoid mutant egg can partially rescue the anterior defects, leading to the development of some anterior structures.
Gain of function (ectopic expression): Injecting bicoid mRNA or anterior cytoplasm into the middle or posterior of a wild-type or mutant egg can induce ectopic head and thoracic structures at the site of injection, often with mirror-image duplications. This shows Bicoid can specify anterior fate in a concentration-dependent manner.
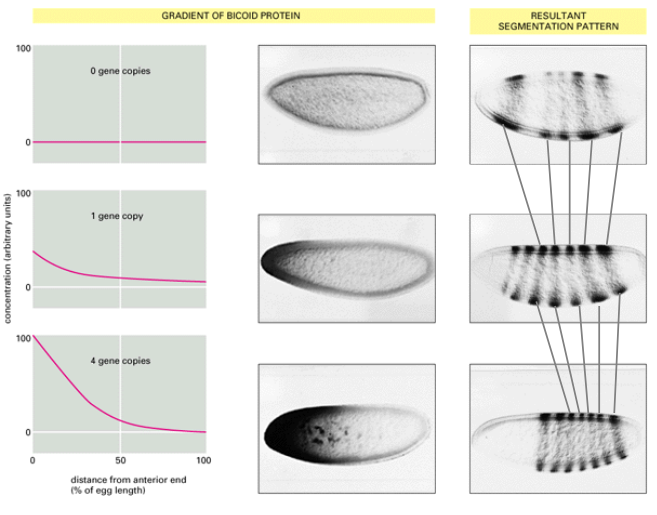
How does the Bicoid protein gradient link to segmentation patterns, and how can varying Bicoid gene dosage affect this?
What does the bicoid protein gradient activate?
What do different levels of bicoid activate?
What does increasing the gene dosage of bicoid from 1 to 4 copies in the mother do?
What happens to structureswhen there is more bicoid?
What does this demonstrate about bicoids role?
The Bicoid protein gradient activates downstream target genes (like gap genes) in a concentration-dependent manner.
Different concentrations of Bicoid activate different sets of genes or activate the same gene at different levels or times.
Increasing the gene dosage of Bicoid (e.g., from 1 to 4 copies in the mother) shifts the boundaries of gap gene expression and subsequently the segmentation pattern anteriorly or posteriorly. In this case it shifted the segmentation pattern more posteriorly.
For example, with more Bicoid, structures normally found more posteriorly will form more anteriorly, and anterior structures will expand.
This demonstrates the morphogenetic role of Bicoid in setting up the body plan.
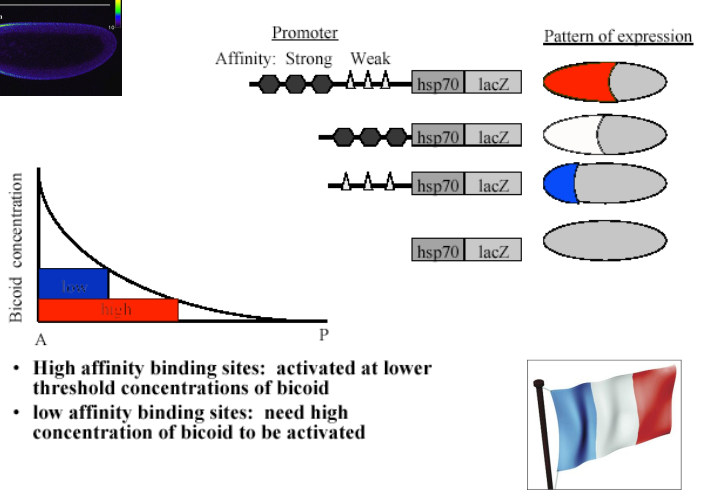
How does Bicoid, as a DNA binding transcription factor, regulate target gene expression based on its concentration?
Bicoid protein binds to specific DNA sequences (Bicoid binding sites) in the promoter/enhancer regions of its target genes.
The affinity of these binding sites for Bicoid protein can vary.
High-affinity binding sites can be activated by lower concentrations of Bicoid. Low-affinity binding sites require higher concentrations of Bicoid to be activated.
This differential affinity allows different genes (or different enhancers of the same gene) to be turned on at distinct positions along the Bicoid gradient, creating stripes or domains of gene expression.
This is sometimes referred to as the "French Flag Model" where different concentrations of a morphogen elicit different cellular responses.
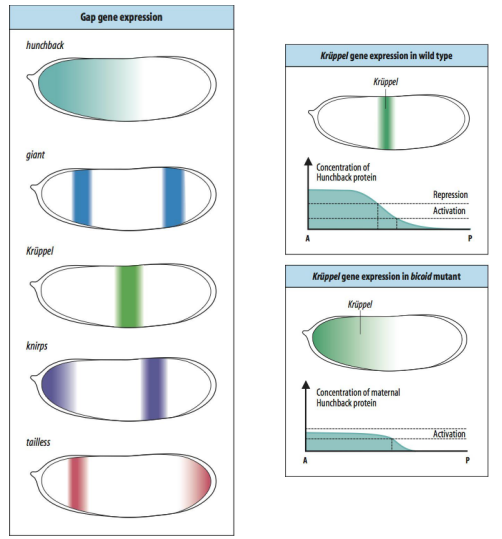
How do maternal genes activate Gap genes, and what is the role of Gap genes?
Maternal gene products, like the Bicoid protein gradient, directly regulate the expression of Gap genes.
Different concentrations of maternal morphogens activate or repress specific Gap genes (e.g., hunchback, Krüppel, knirps, giant, tailless).
Gap genes are expressed in broad, overlapping domains along the A-P axis.
Their role is to interpret the maternal gradients and further subdivide the embryo into coarser regions, defining "blocks" or domains of gene expression that will later give rise to groups of segments.
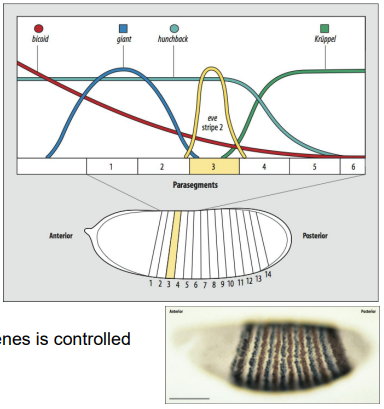
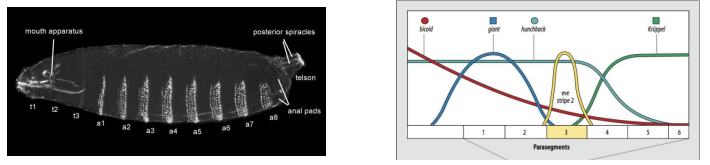
How do Gap genes activate Pair-rule genes, and how is their striped expression controlled?
As an example, what is eve stripe 2 activated by, and what is it repressed by?
Combinations of Gap gene proteins (acting as both activators and repressors) and maternal factors regulate the expression of Pair-rule genes (e.g., even-skipped (eve), fushi tarazu (ftz)).
Each stripe of a pair-rule gene is typically controlled by its own specific enhancer element that responds to a unique combination of upstream transcription factors (Gap proteins and maternal factors).
For example, eve stripe 2 is activated by Bicoid and Hunchback, and repressed by Giant and Krüppel.
This complex combinatorial control results in the characteristic seven-stripe pattern of pair-rule gene expression, defining the 14 parasegments.
How do Pair-rule genes activate Segment polarity genes, and what is their role in refining patterning?
Pair-rule gene products, in turn, regulate the expression of Segment polarity genes (e.g., engrailed (en), wingless (wg), hedgehog (hh)).
Segment polarity genes are expressed in narrow stripes within each of the 14 parasegments, typically one or two cells wide.
They establish and maintain the boundaries between parasegments (and later segments) and define the anterior-posterior polarity within each segment.
They often participate in signalling loops (e.g., Wg and Hh) to stabilize these patterns.
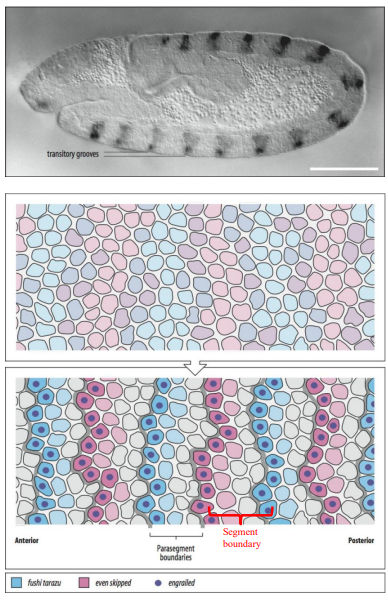
What are parasegments, and how do they relate to the final segments of a Drosophila larva? Give examples of segment polarity genes involved.
Parasegments are the primary, repeating developmental units established by pair-rule genes in the Drosophila embryo.
There are 14 parasegments.
Each parasegment consists of the posterior compartment of one future segment and the anterior compartment of the next segment.
For example, the segment polarity gene engrailed (en) marks the anterior boundary of each parasegment (which becomes the posterior compartment of a segment), while wingless (wg) is expressed in the cells just anterior to engrailed. Hedgehog (hh) is expressed in the engrailed-expressing cells.
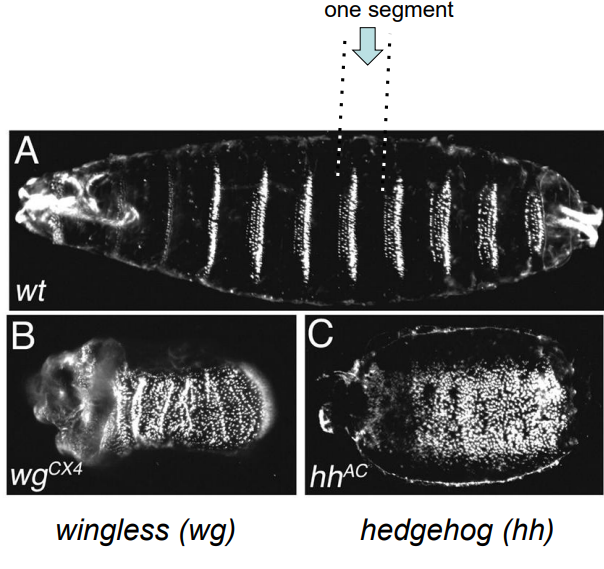
These genes interact to establish and maintain parasegment boundaries, which then lay the coordinates for the future segments. (e.g., wgCX4 and hhAC are mutant alleles of wingless and hedgehog).
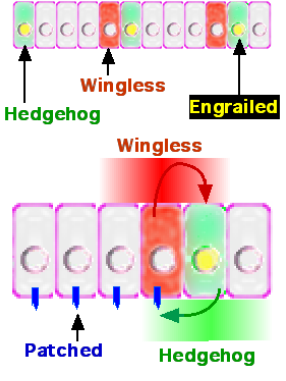
How do interactions between Hedgehog (Hh), Wingless (Wg), and Engrailed (En) establish and maintain parasegment boundaries?
Engrailed (En) is expressed in the anterior-most cells of each parasegment (posterior compartment of future segment).
En-expressing cells secrete Hedgehog (Hh) ligand.
Hh signals to the adjacent anterior cells (anterior compartment of future segment), inducing them to express Wingless (Wg).
Wg ligand then signals back to the En-expressing cells, maintaining En and Hh expression.
This reciprocal signalling loop between Hh and Wg, mediated by their receptors (Patched for Hh, Frizzled for Wg) and downstream pathways, stabilizes the parasegment boundary and refines segment borders.
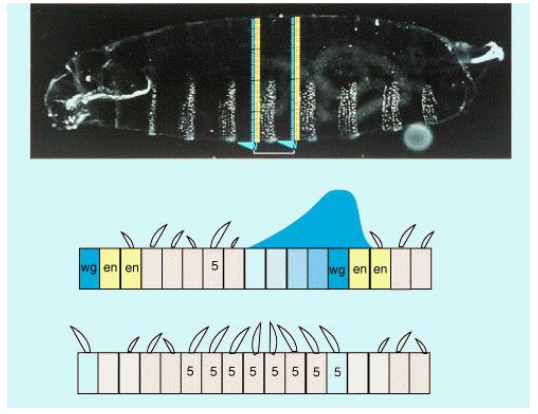
How do segment polarity genes control denticle patterns in the Drosophila larva?
As an example, how does Hedgehog (Hh) signalling impact Wingless (Wg) expression? How does this impact denticle formation?
Segment polarity genes, through their interactions, control the fate of cells within each segment, including which cells will secrete denticles (ventral hairs) and which will form smooth ("naked") cuticle.
For example, Hedgehog (Hh) signaling maintains Wingless (Wg) expression. Wg signaling, in turn, generally suppresses denticle formation in the cells that receive the Wg signal, leading to the "naked" cuticle region. Disruption of this signaling (e.g., in a wg mutant) leads to an expansion of denticle-forming cells.
Selector genes aka homeotic genes
What are selector genes?
What are Hox genes (Homeotic genes)?
What is their role in giving segments identity?
Examples in Drosophila:
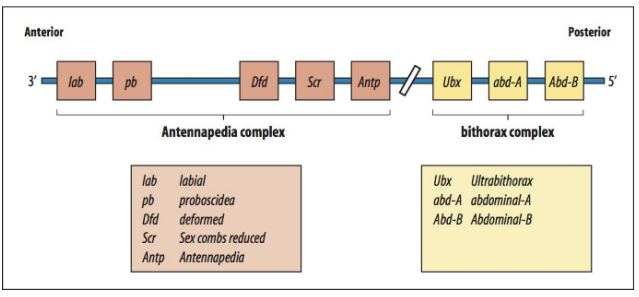
Antennapedia complex genes, specifically Antp. What does Antp specify? What would an Antp mutant do?
What is bithorax complex genes’ expression controlled by?
What does the bithorax complex gene Ultrabithorax (Ubx) specify?
What does it mean for Hox genes to have colinearity?
What is the highly conserved DNA-binding domain contained within Hox genes?
Selector genes act as "master switches" that determine the identity of body segments and structures.
Hox genes (also called homeotic selector genes) are a family of transcription factors that provide "identity" information to each segment along the anterior-posterior axis.
They determine what structures will develop in a particular segment (e.g., legs, wings, antennae).
Examples in Drosophila include:
Antennapedia complex genes (Antp) - normally specifies leg identity, mutant causes legs to grow where antennae should be.
Bithorax complex genes’ expression is controlled by a combination of gap and pair-rule gene products.
Ultrabithorax (Ubx)- specifies posterior thoracic and anterior abdominal identity.
A key feature is colinearity: the order of Hox genes on the chromosome often mirrors their order of expression along the A-P axis.
They contain a highly conserved DNA-binding domain called the homeobox.
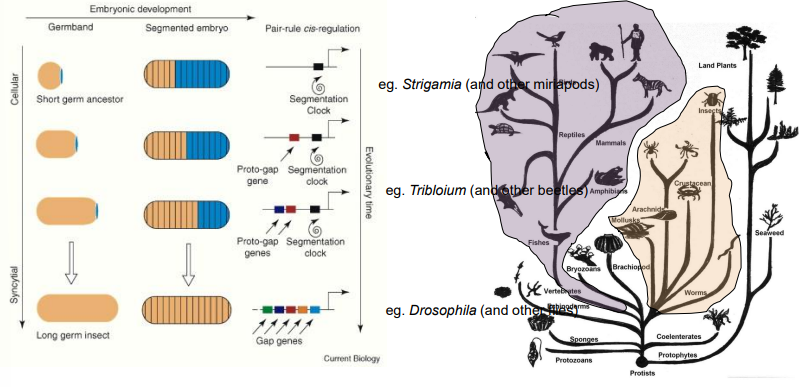
Compare and contrast "long germ band," "short germ band," and "intermediate germ band" insect development.
Long germ band insects (e.g., Drosophila):
All segments (e.g in Drosophial all 14 segments are defined at once) are specified simultaneously or very early in development.
Largely through the action of maternal and zygotic gene hierarchies acting across the entire embryo.
Embryogenesis is often rapid (e.g., 24 hours in Drosophila).
The system is complex due to interactions for every segment - maternal, gap, pair rule genes interact for every segment.
Short germ band insects (e.g., Tribolium beetle, grasshoppers):
Only the anterior segments (head and thorax) are specified initially.
Posterior (abdominal) segments are added sequentially from a posterior growth zone/posterior disc (proctodeum), which buds off segments as it gets smaller.
This is thought to be a more ancestral version of the system Drosophila now uses. Likely representing the original ‘ancestral’ segmentation mechanism.
Has moderate complexity but slower.
Intermediate germ band insects:
Show features of both, with some anterior segments specified early and posterior ones added sequentially.
How are segments added in short/intermediate germ band insects like Tribolium?
In short/intermediate germ band insects, after the initial head and thoracic segments are laid down (likely via an ancestral version of the Drosophila system), abdominal segments are added sequentially.
A posterior region, often called the proctodeum or posterior disc, acts as a growth zone, "budding off" new segments as it gets smaller.
This sequential addition is often slower and considered to represent a more ancestral segmentation mechanism.
The ‘Segmentation Clock’ Mechanism
How are segments added in the invertebrate Strigamia maritima (centipede) and many vertebrates?
Describe the "segmentation clock" mechanism, and the key components.
What is the communication of Notch-Delta signalling?
What are Hes/Her genes?
How do the negative feedback loops work?
What does Notch-Delta signalling activate?
What are Hes/Her proteins, and what do they do? What does this form?
How does the feedback loop set the period for segment formation?
How are the oscillations synchronised across cells (hint: wave)?
What ultimately defines the segment boundaries?
In Strigamia and many vertebrates (for somitogenesis), segments are added sequentially from a posterior growth zone via a "segmentation clock." The segmentation clock governs the rhythmic formation of body segments (somites) in developing embryos, particularly in vertebrates like Zebrafish and invertebrates like Strigamis (centipedes).
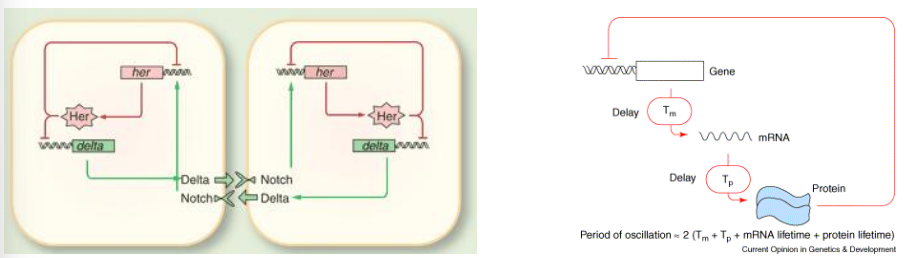
The segmentation clock operates through a series of molecular oscillations within the pre-somitic mesoderm (PSM), the unsegmented tissue from which somites are formed. A core component of this clock involves a crucial negative feedback loop centered around the Notch signaling pathway and a family of genes called Hes/Her.
Notch-Delta signaling: Cells communicate via Notch receptor and Delta ligand.
Hes/Her genes are homologues of the Hairy/Enhancer of split genes in Drosophila).
Negative feedback loops:
Notch-Delta signaling activates Hes/Her gene transcription in receiving cells.
Hes/Her proteins are repressors that downregulate their own transcription and that of Delta, forming a negative feedback loop.
This feedback loop, with a time lag, causes oscillations between strong and weak signaling levels, setting the period for segment formation (the period of ocsillation- one cycle - determines the time it take to form one segement).
The oscillations are synchronized across PSM cells via the Notch-Delta pathway. As Hes/Her levels drop, Delta expression rises, activating Notch in neighbouring cells and creating a wave of activateion (gene expression).
This anteriorly propagating wave of activation from the oscillating PSM ultimately defines segment boundaries.
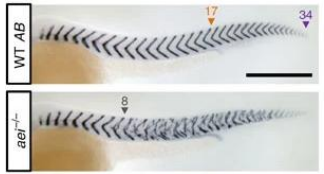
Describe how the segmentation clock was evidenced through an experiment on Zebrafish somitogenesis (using aei/DeltaD mutants).
Studies in zebrafish somitogenesis, such as those involving the after eight (aei) mutant, provide strong evidence for this mechanism.
The aei gene encodes DeltaD, a Notch ligand.
Mutants for aei/DeltaD exhibit severe segmentation defects, highlighting the critical role of Delta-Notch signaling in maintaining the synchronized oscillations necessary for proper somite formation.
The disruption of this signaling pathway leads to a loss of the rhythmic patterning.
Segmentation in Vetebrates
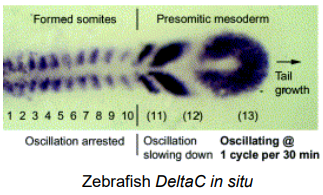
Which genes are cycling genes (have oscillatory expression)?
What is the “Pacemaker” of the segmentation clock?
What does the presomitic mesoderm (PSM) extending posteriorly show about where the segmentation clock is active?
Cycling Genes have Oscillatory Expression within the PSM indicating their involvement in the segmentation clock.
Notch Signaling Components
Hairy/Enhancer of Split (Hes/Her) Related Transcription Factors
Wnt/β-catenin pathway components
The "Pacemaker": The majority of known candidate pacemaker genes lie in the Notch pathway. While multiple pathways contribute, the Notch signaling components and their downstream effectors (Hes/Her genes) are central.
PSM (Presomitic Mesoderm) is unsegmented tissue extending posteriorly from the formed somites. This is where the segmentation clock is active. The oscillating clock mechanism progressively defines new segment boundaries as the embryo elongates. This can be visualised via the in situ hybridisation of Delta.
What is the evolutionary relationship between the segmentation mechanisms seen in Drosophila (long germ), Tribolium (short/intermediate germ), and Strigamia (myriapod)?
Drosophila's "long germ band" development, where all segments are patterned nearly simultaneously, is considered a derived (more evolved) state, likely adapted for rapid development. It does not involve the segmentation clock mechanism.
Short/intermediate germ band development (e.g., Tribolium) and the sequential segment addition seen in myriapods like Strigamia (using a Notch-Delta based segmentation clock) are thought to represent more ancestral mechanisms.
The evolutionary trend in some insect lineages (like Diptera, which includes Drosophila) has been towards condensing and overlapping these processes for speed.
The underlying molecular components (like Notch signaling) are ancient, but their deployment in "simultaneous" vs. "sequential" modes has diverged.