Alkanes & Cycloalkanes
1/58
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
59 Terms
Organic Chemistry Reactions
Follows either of the two common general reaction mechanisms; Polar Mechanism & Radical Mechanism
Polar Mechanism
It results from a heterolytic bond cleavage/formation.
This is a more common reaction mechanism because of the interaction between; Nucleophile & Electrophile
Nucleophile (Nü)
neutral/negatively charged atom (e.g. HO-, H2O).
Electrophile (e+)
neutral/positively charged atom (e.g. C=O, R-X
Radical Mechanism
It results from a homolytic bond cleavage/formation.
This is a common reaction mechanism for alkanes/cycloalkanes in which a radical (an atom with a single unpaired electron) is formed at high temperature (𝚫) or irradiation (hv)
Radical
an atom with a single unpaired electron
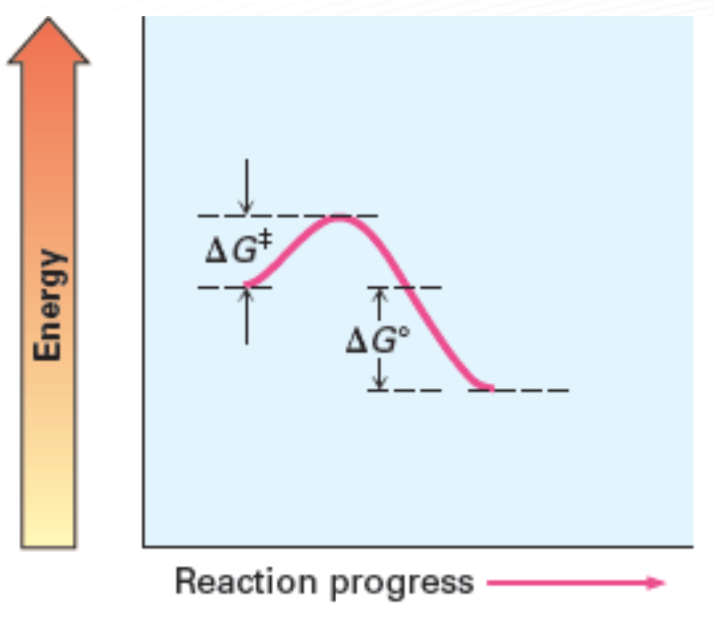
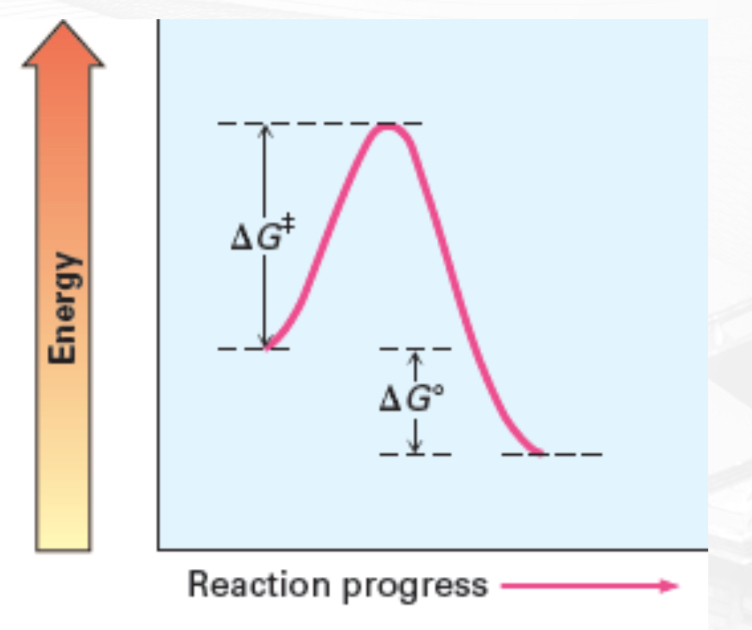
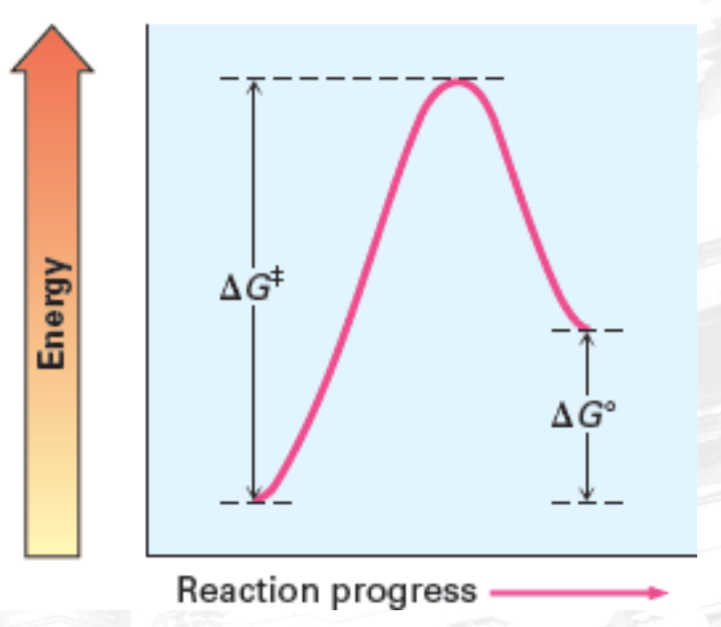
Activation Energy (Ea)
Minimum energy required to reach the transition state
Transition state (Ts)
A state of peak free energy and where reactants starts to form products.
Delta G naught prime (𝚫G°)
a free energy change of a reaction in standard condition
Fast/Slow
refers to the amount of Ea to reach Ts; inversely proportional with Ea.
Exergonic reaction
reactions that releases energy; has a negative 𝚫G°
Endergonic reaction
reactions that absorbs energy; has a positive 𝚫G°
with a negative 𝚫G°
Fast and Exergonic
with a negative 𝚫G°
Slow and Exergonic
with a positive 𝚫G°
Fast and Endergonic
with a positive 𝚫G°
Slow and Endergonic
Fast, Exergonic, negative ∆G○
Slow, Exergonic, negative ∆G○
Fast, Endergonic, positive ∆G○

Slow, Endergonic, positive ∆G
carbocation intermediate
require energy for activation in order to progress thru the reaction
Alkanes
General formula: CnH2n+2
These organic compounds are referred to as saturated hydrocarbons (HC). It is made up of completely single bonds.
Petroleum and natural gas
sources of alkanes and a mixture of hydrocarbons
Kinds of alkanes
linear-chain / normal (n) and branched-chain alkanes
Cycloalkanes
General formula: CnH2n
Similar to alkanes, they are also saturated hydrocarbons (HC) but has 2 fewer hydrogens compared to normal alkanes.
Have their terminal carbons connected to created an enclosed cyclic system.
They can be substituted with different atoms or functional groups similar to a normal alkane
Linear-Chain Alkane
n-pentane
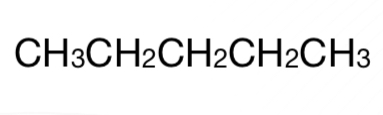
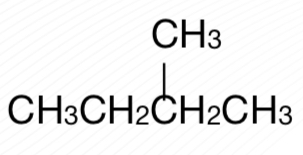
Branched-chain alkane
2-methylbutane
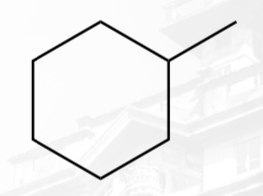
monosubstituted cycloalkane
methylcyclohexane
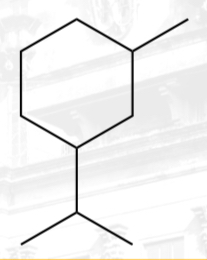
disubstituted cycloalkane
1-isopropyl-3-methylcyclohexane
Petroleum and natural gas
sources of alkanes and a mixture of hydrocarbons.
Alkanes & Cycloalkanes
This is insoluble in water
Alkanes & Cycloalkane
Both of them exhibits London dispersion forces which is a non-polar IMF and is not soluble in polar molecule such as water.
Lower density
Alkane & Cycloalkane this is the density in water.
Dissolve in water
This what happens to alkanes & cycloalkanes in water & would float
Boiling points of linear alkanes and cycloalkanes increases
This is as the carbon chain increases in length or size.
the number of IMF (intramolecular forces) increases
As the carbon chain increases this what happens
It is required to be broken for a material to boil.
Alkanes & Cycloalkanes
Least reactive of all organic compounds but are not completely unreactive
Combustion & Cracking
This reaction undergoes in Alkane & Cycloalkanes
Combustion
Reaction between a susbtance and O2 that produces CO2 and H2O resulting in the production of large amount of heat and light.
Cracking
Refers to a chemical process where large hydrocarbon molecules (like long-chain alkanes or cycloalkanes) are broken down into smaller, more useful molecules like alkenes and shorter alkanes with the aid of heat and/or Al2O3 catalyst
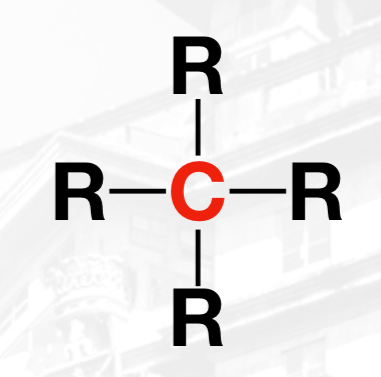
Stability increases from primary to quaternary
The stability——— primarily because the R-groups provides support on stability when a carbocation is formed
Primary Carbon
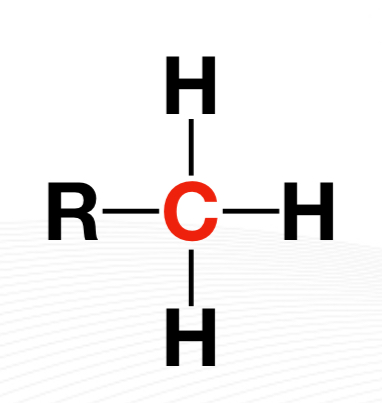
Secondary Carbon
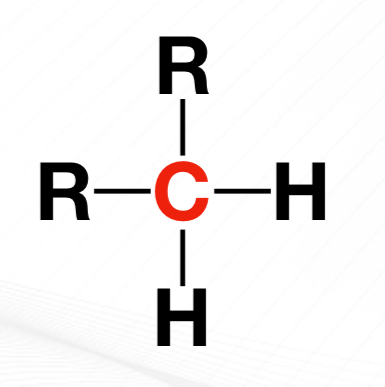
Tertiary Carbon
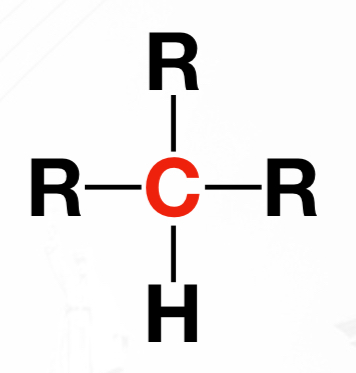
Quaternary carbon
Substitution-type reaction.
Alkanes and Cycloalkanes will only react under specific conditions of temperature and irradiation following this…
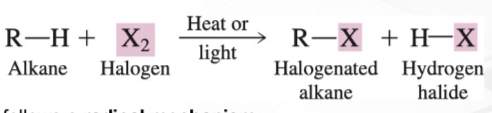
Substitution Reactions
Chemical reaction in which part of a small reacting molecule replaces an atom or a group of atoms on a hydrocarbon or hydrocarbon derivative.
Halogenation
Chemical reaction between a substance and a halogen in which one or more halogen atoms are incorporated into molecules of the substance
Radical Mechanism
Radical Mechanisms
R-H (alkane/cycloalkane) demonstrates the -H atom that will be replaced by one incoming -X atom.
R-X (halogenated alkanes / haloalkanes
Primary product/formation of radical mechanism
H-X as a by-product
The -H and -X that was cleaved from their respective reactants will form this
Radical/Free radical
Atom with an unpaired electron
Initiation Phase
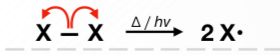
Propagation Phase
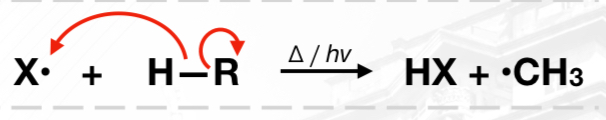
Termination Phase
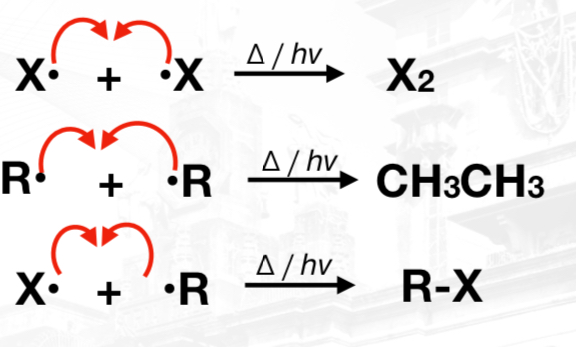
mono- , di-, or poly- substituted products
This is formed by radical substitution as halogenated alkane can continuously react with halogens resulting to polyhalogenated products.
Excess (x’ss) amount of alkane
This is used to maximize monohalogenation and prevent continuous propagation of radical species
Reactivity Selectivity Principle
States that the greater the reactivity of the species, the less selective it will be
This is applied where there are multiple carbons that can undergo halogenation
It aids in determining the most common halogenation product that can be formed.