AP BIO 1.6 - 1.7
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Nucleic acids
constitute the genetic material of living organisms
DNA and RNA
store and transmit genetic information, regulatory functions
No Sulfur
Covalent bonds, called phosphodiester bonds, are formed between the 3’ Carbon of the terminal sugar and the phosphate group of the nucleotide
Nucleotides
monomers for nucleic acids
consists of: phosphate, nitrogen base, sugar (ribose or deoxyribose)
joined by condensation synthesis
Nitrogen bases
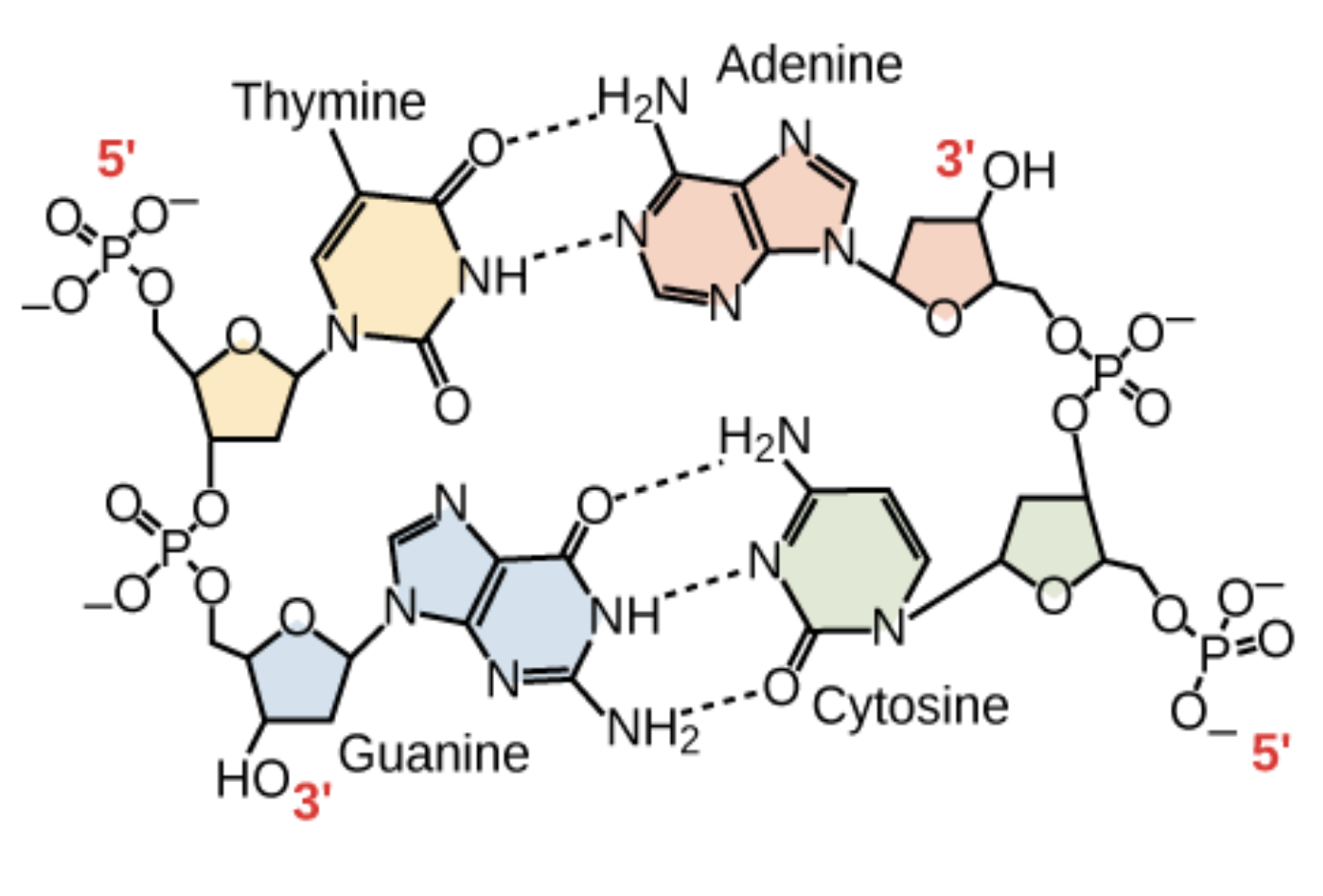
Thymine = Adenine (ONLY DNA)
Uracil = Adenine (ONLY RNA)
Guanine ≡ Cytosine
Purines (2 rings): Adenine and Guanina
Pyramadines (1 ring): Thymine Cytosine Uracil
DNA
deoxyribose nucelic acid
has deoxyribose
sugar lost the O in the prime carbon
the bases are joined via hydrogen bonds
one strand runs 5′ to 3′
the other 3′ to 5′
RNA
ribose nucleic acid
has ribose
sugar has not lost the O
usually single-stranded, but may be folded into 3-D structures via H-Bonds
Nucleic acid structure
The sugar and phosphate lie on outside of helix
Nitrogenous bases are stacked in the interior
The strands of the helix run in opposite directions (antiparralel orientation)
Each base from one strand interacts via hydrogen bonding with a base from the opposing strand
proteins
Major functions of proteins:
Enzymes—catalytic proteins
Defensive proteins (e.g., antibodies)
Hormonal and regulatory proteins—control physiological processes
Receptor proteins—receive and respond to molecular signals
Storage proteins store amino acids
Structural proteins—physical stability and movement
Transport proteins carry substances (e.g., hemoglobin)
Genetic regulatory proteins regulate when, how, and to what extent a gene is expressed
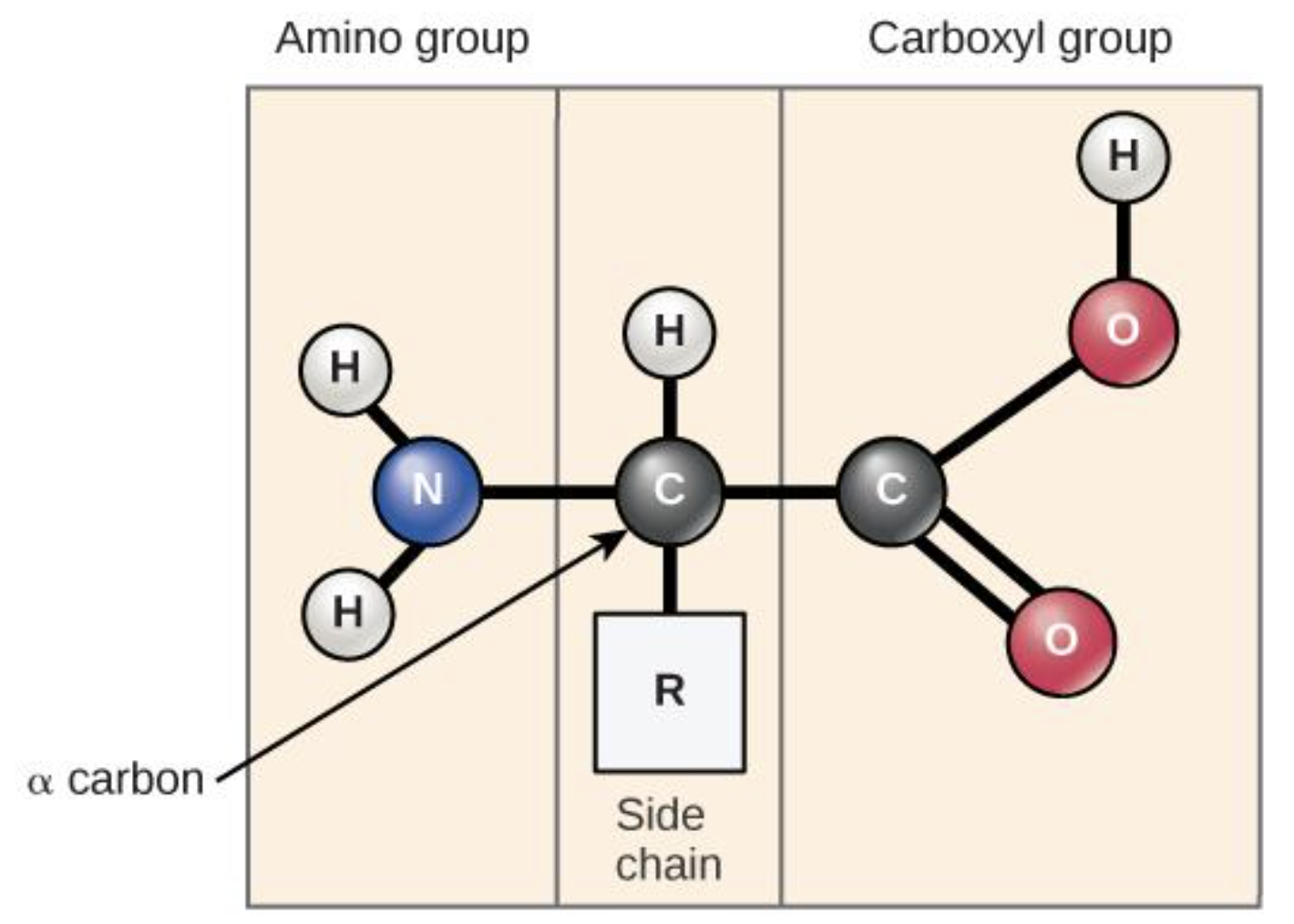
Amino Acids
monomes that makes porteins
tRNA grabs amino acids to create proteins
Amino group on the left connected to an alpha carbon atom that is connected to carboxyl group on the right and a H atom above the carbon
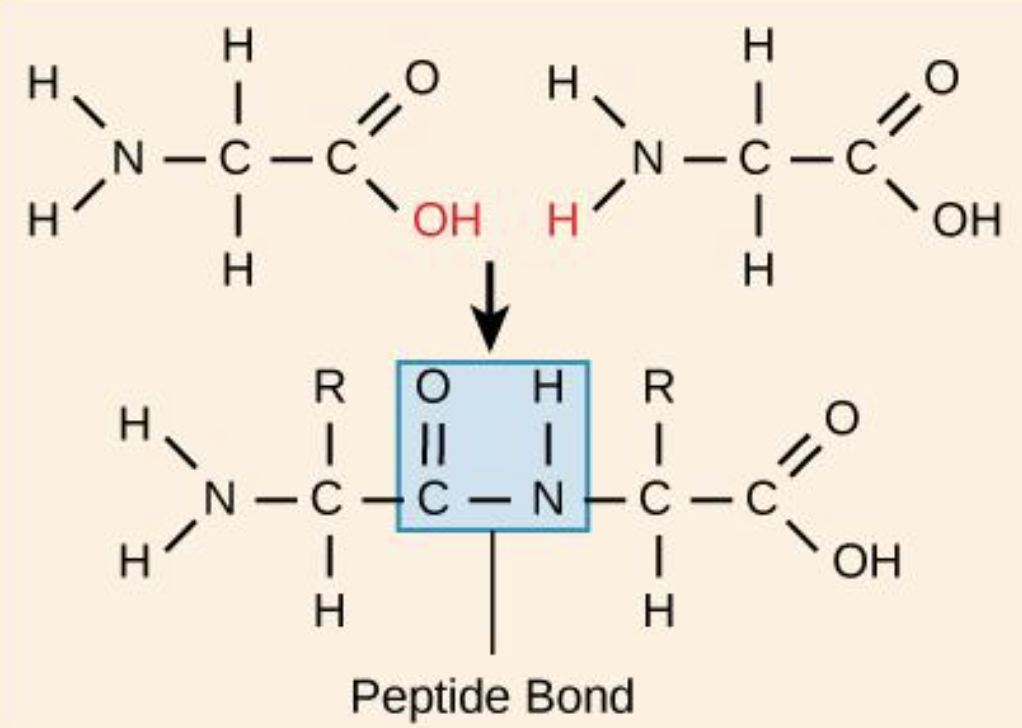
How are amino acids linked?
via peptide bond/linkage, a dehydration synthesis reaction
Carboxyl group of one amino acid is linked to the amino group of the incoming amino acid
A molecule of water is released as part of the reaction
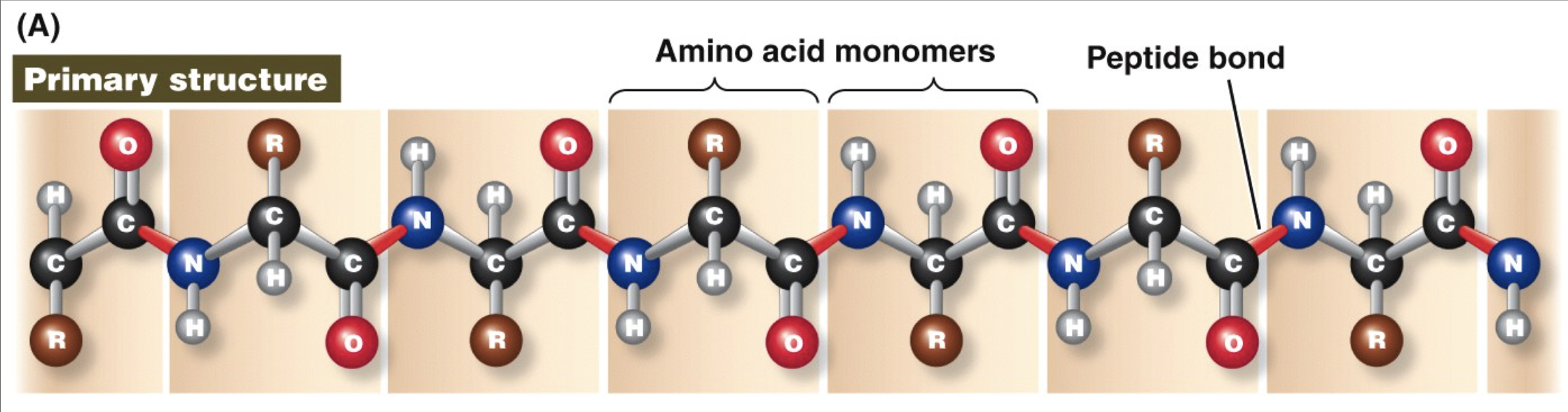
polypeptide
a chain of amino acids joined together by peptide linkages
primary protein structure
sequence of amino acids in a polypeptide
Determined by the message encoded in the nucleotide sequence in DNA.
secondary protein structure
polar amino acids create H Bonds, resulting in repeated spatial patterns in different regions
form because of hydrogen bonding between carbonyl and amino groups in the peptide backbone
alpha helix: right-handed coil
pleated sheet
interaction of peptide backbone
Tertiary
side chain interactions
polypeptide chain is bent and folded
3D shape
outer groups are functional groups that can interact
single polypeptide is formed
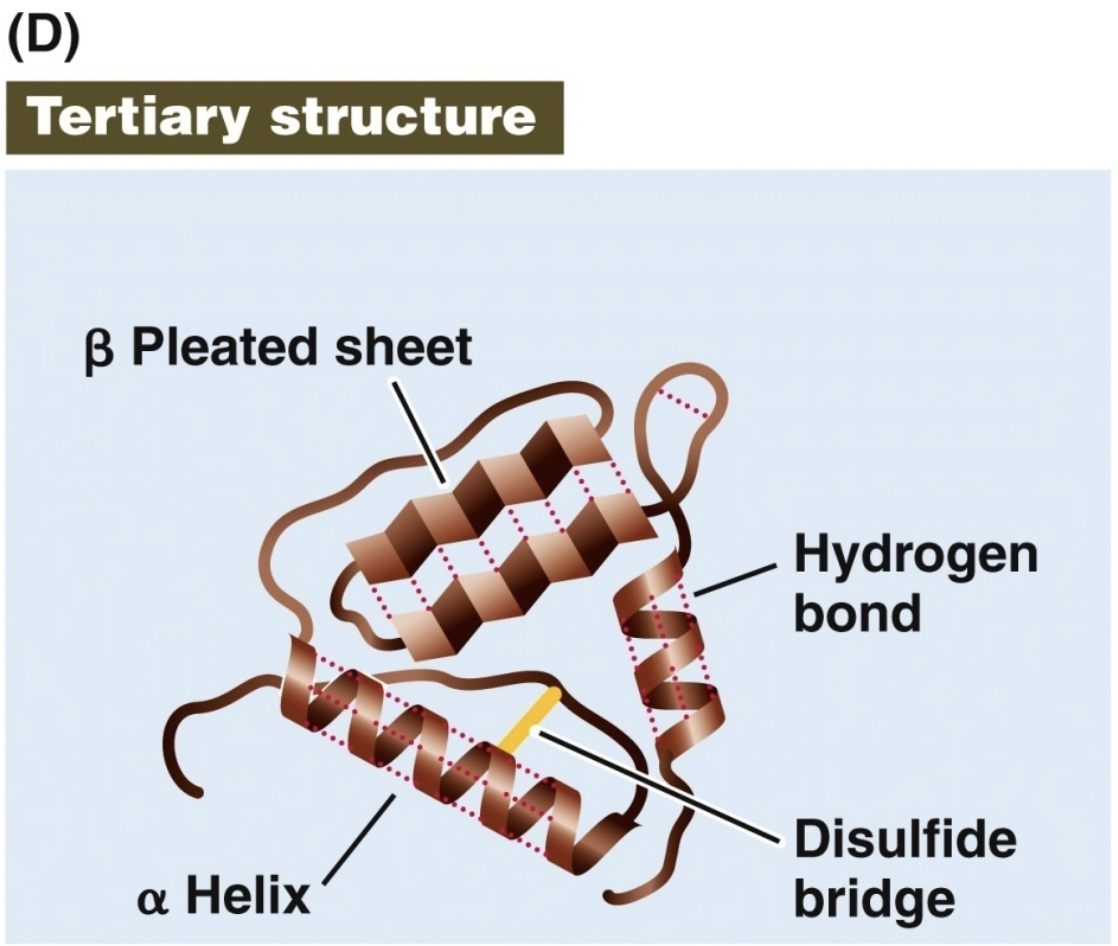
quaternary protein structure interations
Disulfide bridges hold a folded polypeptide together
Hydrogen bonds stabilize folds
Hydrophobic side chains can aggregate
van der Waals interactions between hydrophobic side chains
Ionic interactions form salt bridges
quaternary protein structure
Two or more polypeptide chains (subunits) bind together by hydrophobic and ionic interactions, and hydrogen bonds.
involves more than 1 polypeptide
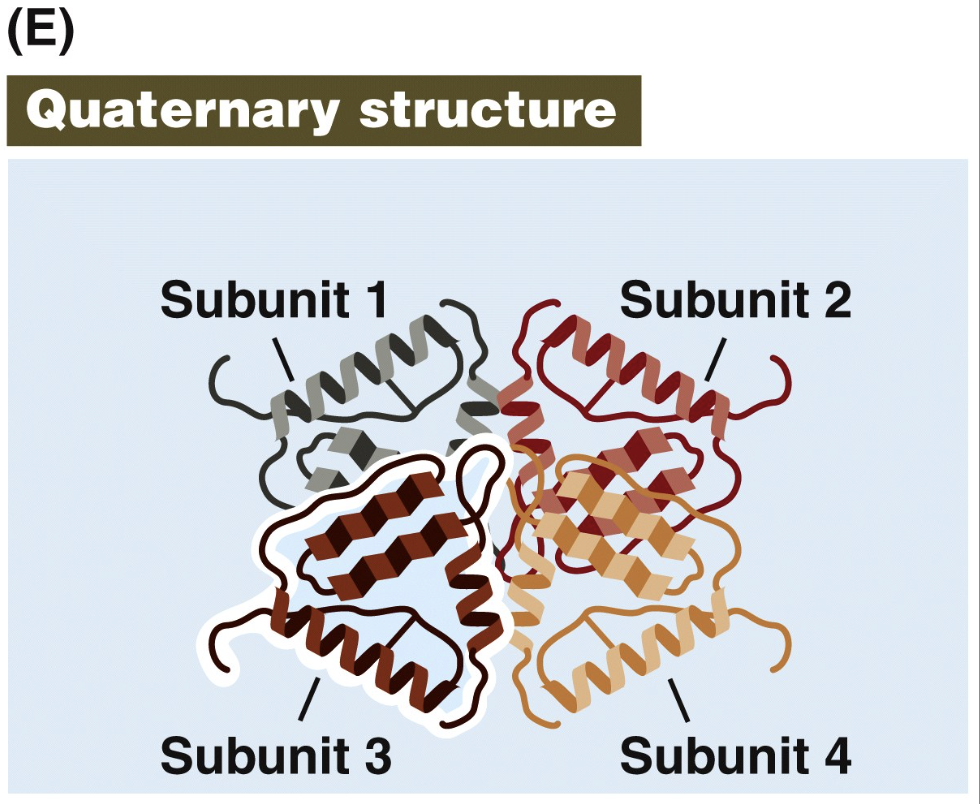
Denaturation
destroying secondary and tertiary structure by disrupting weaker interactions
caused by
High temperature
Change in pH
Change in solvent – polar to nonpolar or nonpolar to polar
Enzymes
catalysts in biochemical reactions
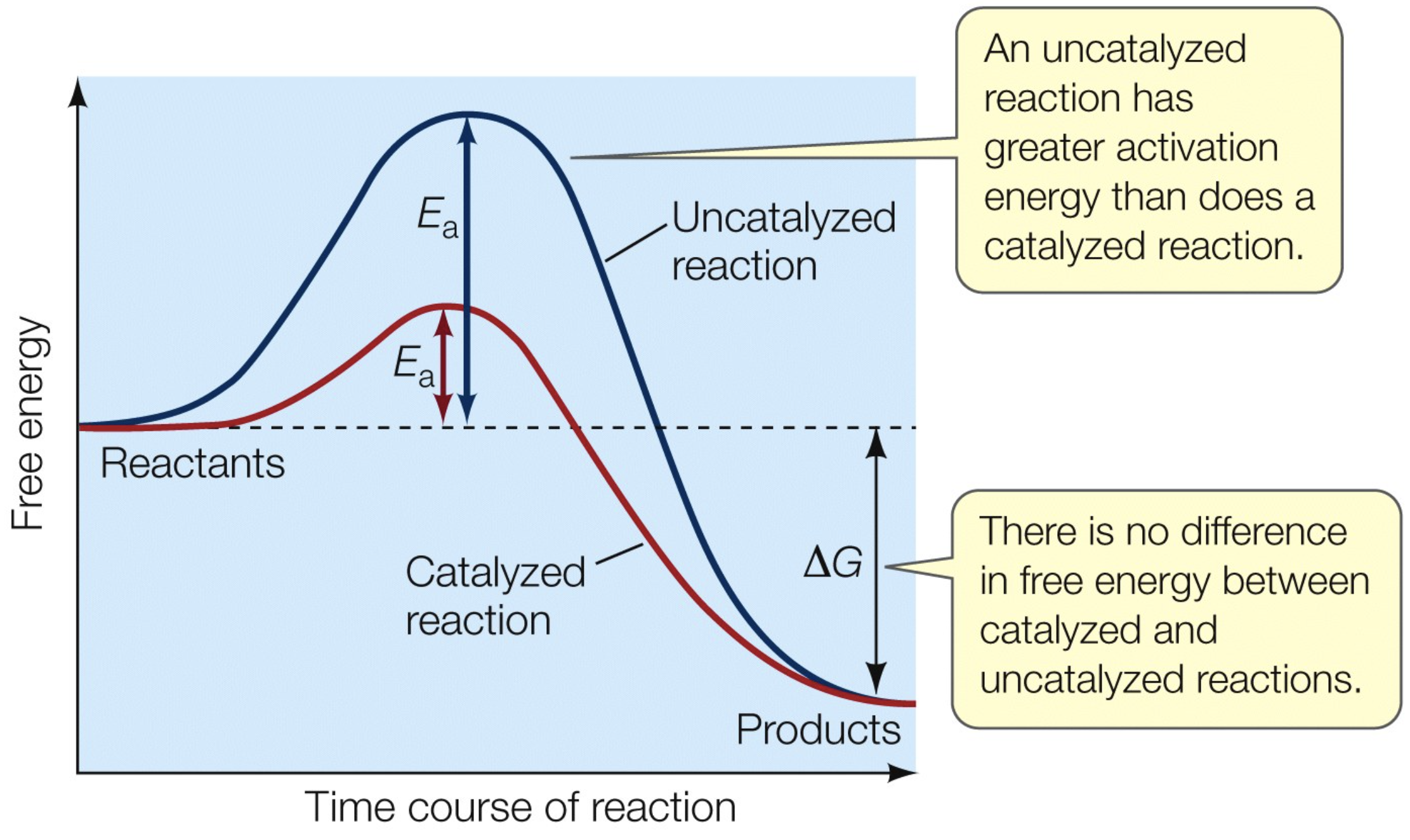
No catalyst makes a reaction occur that cannot otherwise occur
The enzyme is not changed at the end of the reaction.
3 types of enzymes
Catabolic – breakdown substrates
Anabolic – build more complex molecules
Catalytic – affects the rate of reaction
Enzyme effect on activation energy
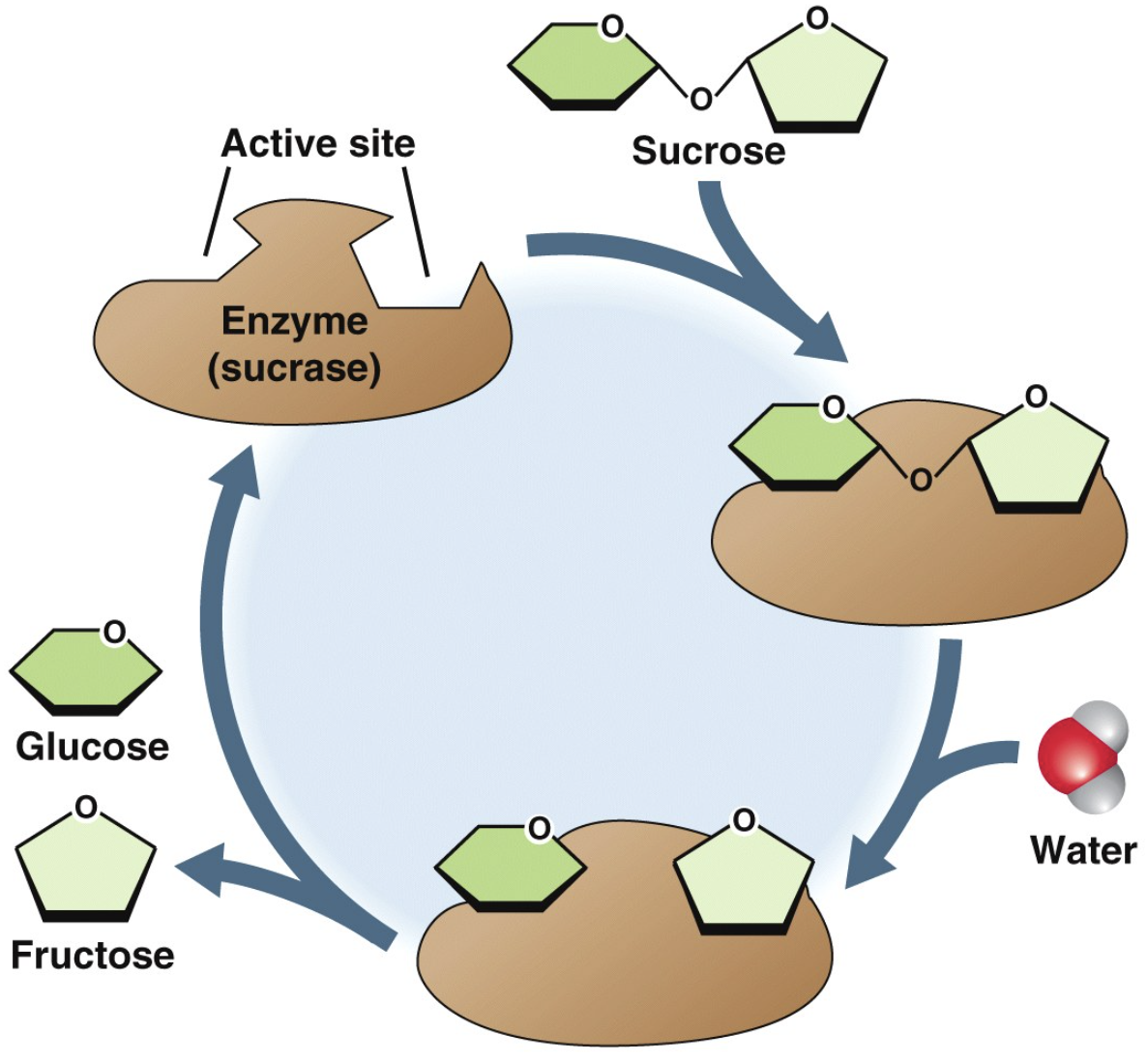
substrates
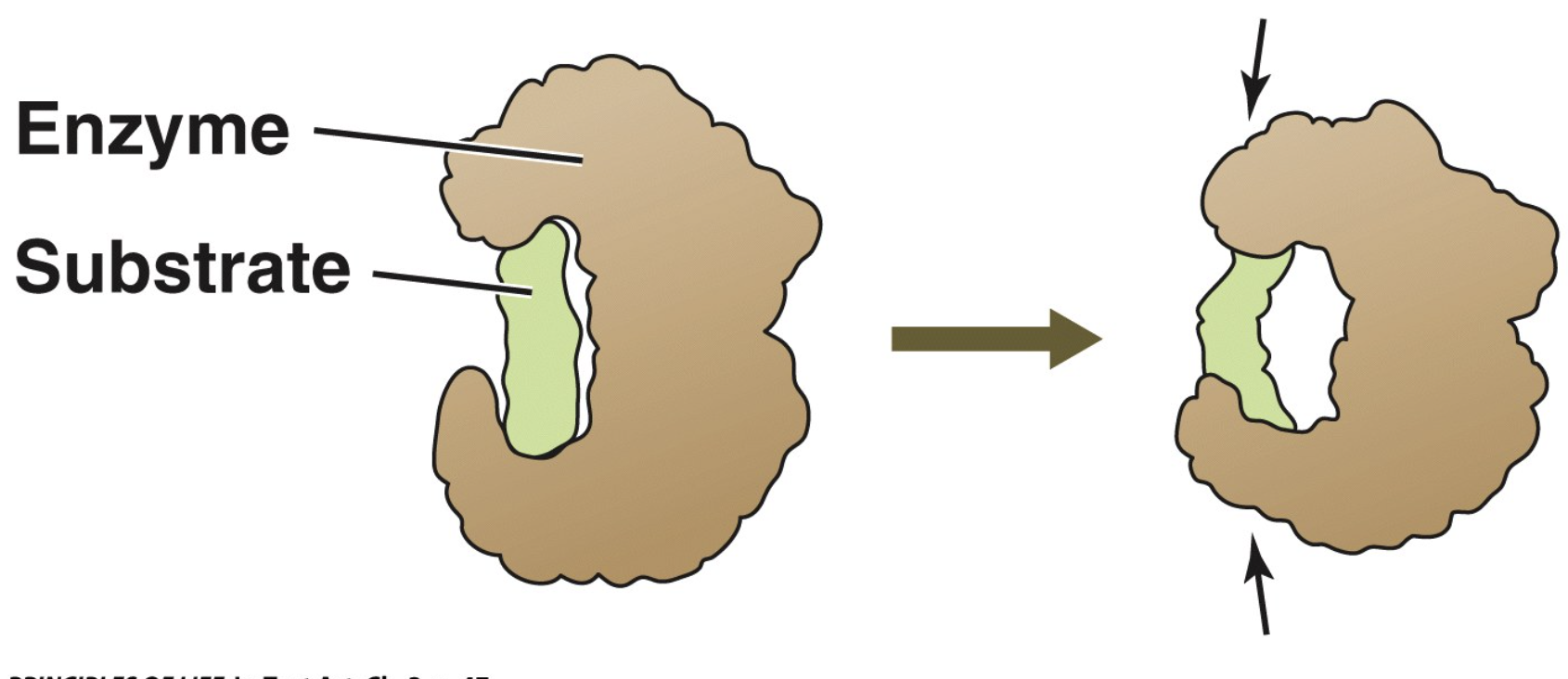
Reactants are substrates: they bind to a specific site on the enzyme—the active site.
The enzyme–substrate complex (ES) is held together by hydrogen bonding, electrical attraction, or temporary covalent bonding.
Enzyme mechanisms
Inducing strain: causing stress and an increase in potential energy within a molecule, material, or biological tissue, often to create a more reactive or useful state, or to study its properties
ions and other molecules required for some enzyme ot function
Cofactors—inorganic ions
Coenzymes add or remove chemical groups from the substrate. They can participate in many different reactions.
Prosthetic groups (non-amino acid groups) permanently bound to their enzymes.
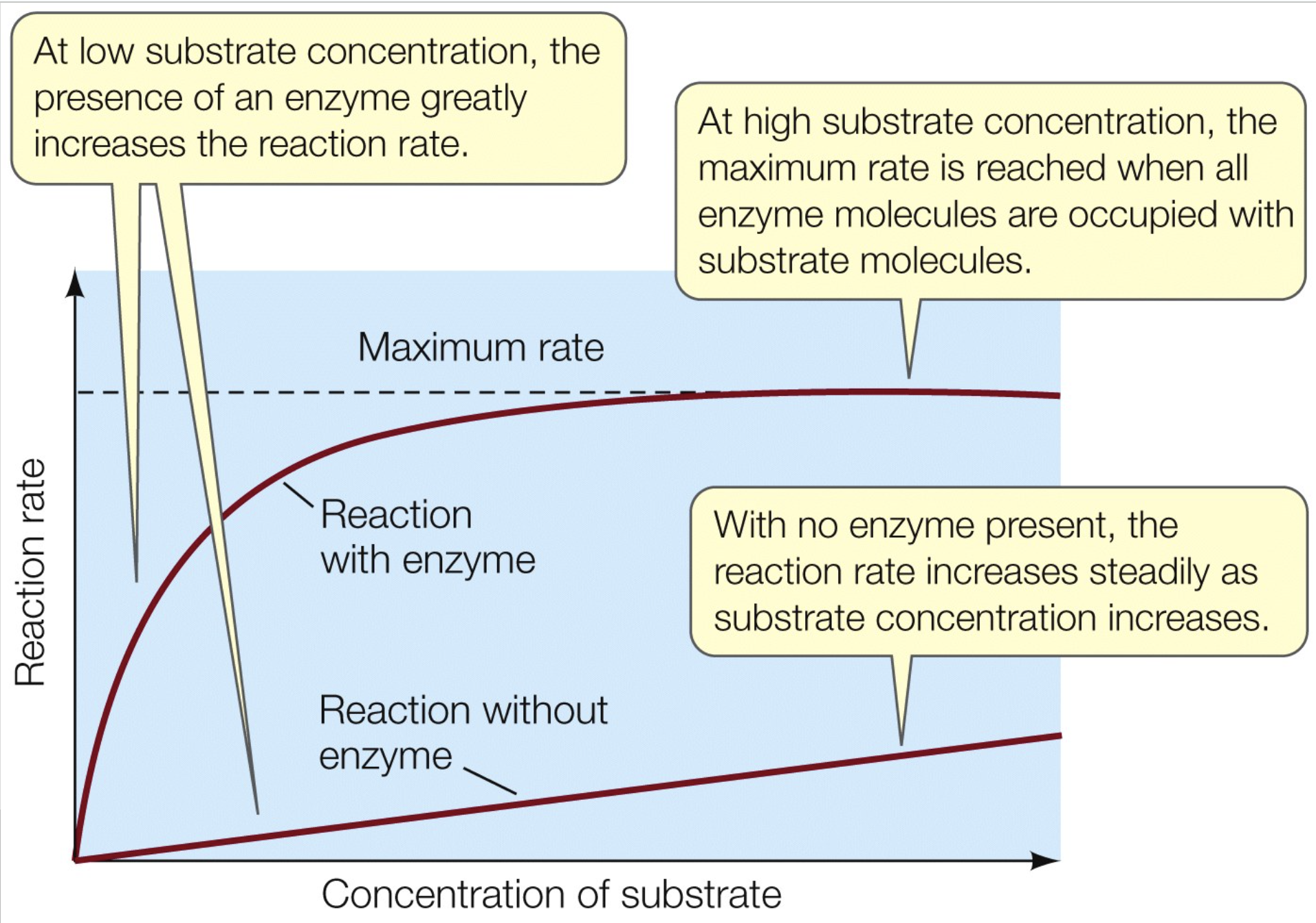
Enzyme effect on rate of reaction
enzyme and homeostasis
Controlling the action of enzymes is an important in metabolic pathways and in homeostasis