exam one
1/74
Earn XP
Description and Tags
ch12, ch13, ch14.1-14.3
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
gas’s shape and volume?
variable shape and volume
liquid’s shape and volume?
variable shape, fixed volume
solid’s shape and volume?
fixed shape and volume
intramolecular forces?
strong
within molecules
covalent and ionic bonds
remains after a physical process
intermolecular forces
weaker
between molecules
affected from physical processes
polar molecule
uneven distribution of charges
permanent dipole moment on molecule
nonpolar molecule
even distribution of charges
no permanent dipole moment on molecule
London dispersion forces
short-lived attractions between instantaneous and induced dipoles
occurs in all molecules because electrons are always moving
larger atoms = stronger LDFs = higher boiling points
strongest in heavier molecules and molecules with greater surface area (therefore linear molecules have stronger LDFs than rings)
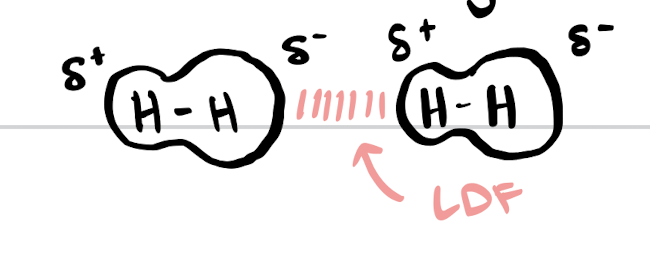
instantaneous dipoles
occur due to asymmetric distribution of electrons
induced dipoles
asymmetric charge in one molecule causes asymmetry in an adjacent molecule
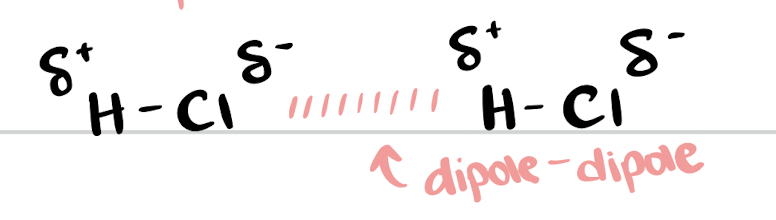
dipole-dipole attractions
electrostatic attractions between oppositely charged ends of polar molecules
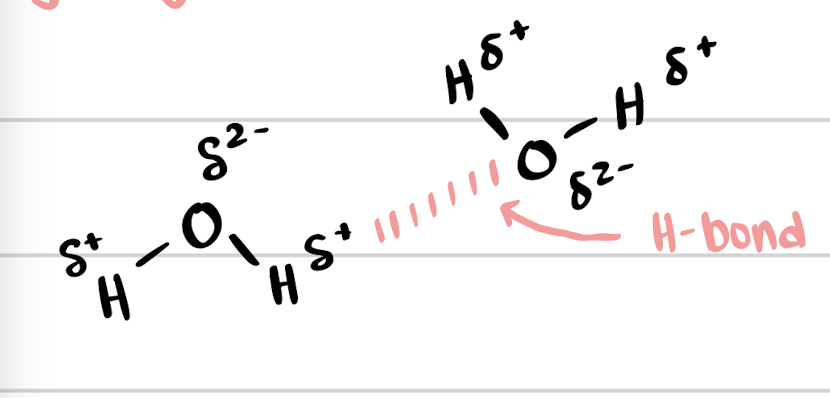
hydrogen bonds (H-bonds)
attraction between partially positive H on one molecules and partially negative F,O,N on another molecule
ion-dipole attractions
electrostatic attraction between an ion and polar molecule
strongest IMF

what does △H stand for in phase changes?
enthalpy change for one mol of the substance
enthalpy = energy absorbed/released during the phase change
+△H
endothermic
energy must be absorbed from surroundings
-△H
exothermic
energy is released to surroundings
name and enthalpy of phase change:
solid → gas
sublimation, +△H
name and enthalpy of phase change:
gas → solid
deposition, -△H
name and enthalpy of phase change:
liquid → gas
vaporization, +△H
name and enthalpy of phase change:
gas → liquid
condensation, -△H
name and enthalpy of phase change:
solid → liquid
melting/fusion, +△H
name and enthalpy of phase change:
liquid → solid
freezing, -△H
heating/cooling curve
shows how temperature changes as pure substance is heated
flat regions = phrase changes
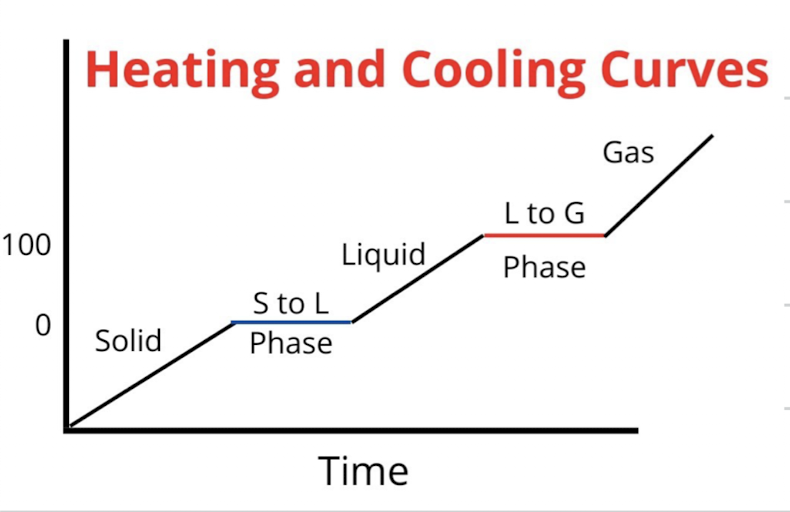
how to calculate energy during a constant phase vs during a phase change
at a constant phase: q = mc△T
during a phase change: q = n△Hvap
(both are not on formula sheet)
vapor pressure definition
pressure of vapor on container walls when at equilibrium
occurs when evaporation + condensation are balanced
what affects vapor pressure?
increasing temperature = molecules move faster = more molecules become vapor = increased vapor pressure
stronger IMFs = harder for molecules to escape phase = lower vapor pressure
weak IMFs = high vapor pressure
boiling point
temperature when vapor pressure of liquid = external atmospheric pressure (equilibrium)
what affects boiling point?
stronger IMFs = higher boiling point because more energy is required to break the bonds
relationship between IMFs, vapor pressure, and boiling point
IMFs ∝ 1/VP ∝ boiling point
NOTE: not a direct linear relationship
surface tension
tendency of a liquid to bead up rather than spread out
viscosity
liquid’s resistance to flow (honey vs water)
what affects surface tension?
strong IMFs = high surface tension
what affects viscosity?
strong IMFs = high viscosity
enthalpy of vaporization formula
Pvap, T = vapor pressure
△Hvap = enthalpy of vaporization
R = universal gas constant = 8.314 J/mol*K
T = temperature (in K)
NOTE: this formula is on equation sheet, but is not given for ACS exam
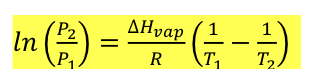
phrase diagram
phase of a substance under all pressure-temperature combinations
x-axis: temperature
y-axis: pressure
critical point on phase diagram
above this point, the Temperature and Pressure conditions allow liquid and gas phase properties to merge and become a supercritical fluid
supercritical fluid
fluid that exhibits has properties/behaves as both a liquid and a gas
triple point
the pressure and temperature where all three phases (solid, liquid, gas) coexist in equilibrium
normal melting point
temperature that solid ↔ liquid equilibrium occurs when pressure = 1 atm
normal boiling point
temperature that liquid ↔ gas equilibrium occurs when pressure = 1 atm
sublimation
solid → gas
deposition
gas → solid
vaporization
liquid → gas
condensation
gas → liquid
melting
solid → liquid
freezing
liquid → solid
solution
any homogeneous mixture
made of almost any 2 phases of matter
solute
the dissolved substance
solvent
the substance that the solute dissolved in
how do you know if a solute will dissolve in the solvent?
like dissolves like → substances dissolve if solute and solvent can form intermolecular attractions
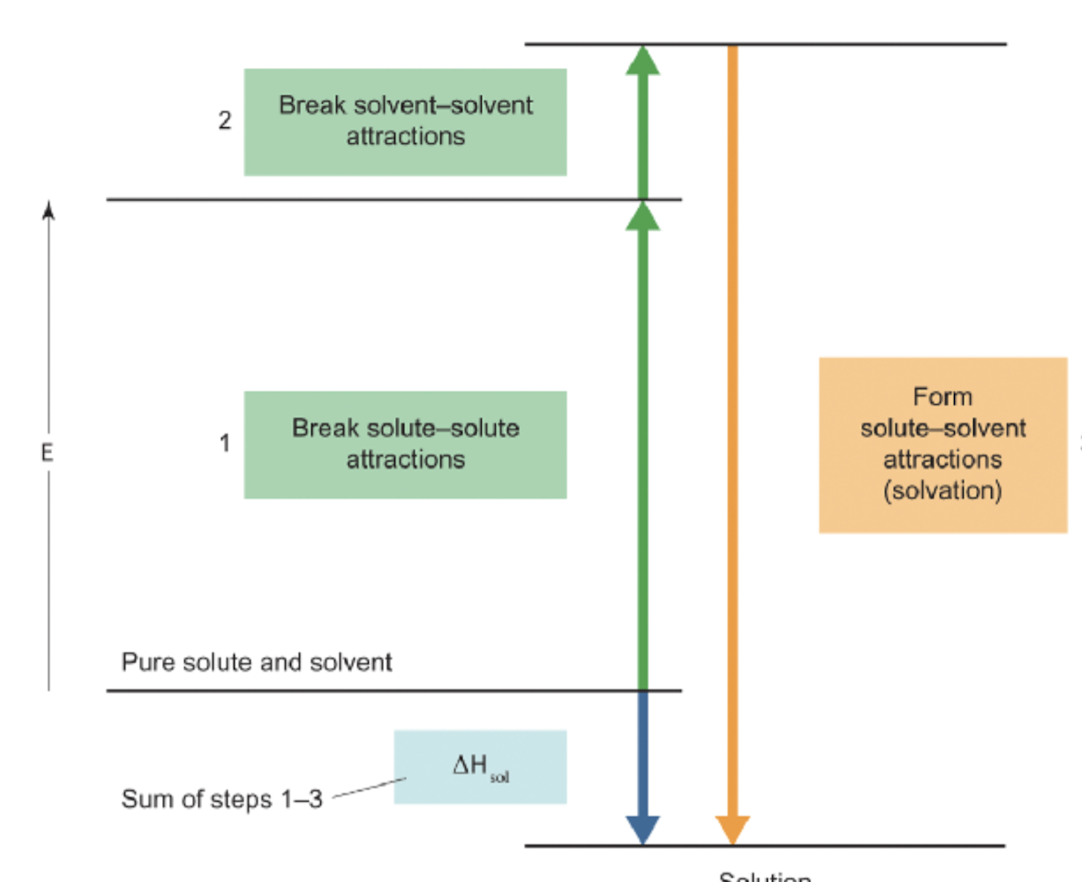
energetics of solution formation
break solute-solute interactions (endothermic, △H1 >0)
break solvent-solvent interaction (endothermic, △H2 >0)
form solute-solvent interactions (exothermic, △H3 <0)
sum of all 3 steps = △Hsol = enthalpy of solution
saturated solution
holds max amount of solute at a given temperature
supersaturated solution
holds more solid than is stable
unsaturated solution
holds less than the maximum amount of solute
relationship between temperature and solubility of gases and solids
as temperature increases, solubility of gases decreases and solubility of solids increase
this is bc KE increases, so more molecules escape into gas phase
Henry’s Law
Cg = kPg
c = solubility of dissolved gas in solution (M)
P = partial pressure of the gas above the liquid (atmosphere)
k = Henry’s law constant (M/atm)
increasing gas solubility = increasing gas partial pressure (direct relationship)
if the temperature is constant, gas solubility is directly proportional to its pressure (so k isn’t needed)
*NOT on equation sheet
percent by mass formula
mass of solute / total mass of solution 100%
*NOT on equation sheet
mole fraction
XA= moles of A / moles of A + moles of B + …
*NOT on equation sheet
molality
m = moles of solute / kg of solvent
*NOT on equation sheet
strong electrolytes
dissociate into ions when dissolved in water
the subscripts in chemical formula show mole ratio
ex: 1.0M AlCl3 contains 1.0M Al3+ and 3.0M Cl-
dissociation of strong electrolytes in water
strong electrolytes fully dissociate in water
dissociation of weak electrolytes in water
weak electrolytes incompletely dissociate in water
dissociation of non-electrolytes in water
non-electrolytes do not dissociate in water
colligative properties
physical properties that change depending on concentration of solute particles
includes boiling point elevation, freezing point depression, vapor pressure depression, osmotic pressure
ex: 1M Na2S(aq) dissolves to Na+, Na+, and S2-, which is 3M ions.
colligative properties don't just depend on molarity, it depends on the number of particles in solution. having more particles = stronger effect on colligative properties
vapor pressure depression equation
Raoult’s Law: Psolution = Xsolvent * P°solvent
Psolution = mole fraction of the solvent
P°solvent = vapor pressure of pure solvent
boiling point elevation
△Tb=Kbm
freezing point depression
△Tf=Kfm
how to find final boiling/freezing point of solution
Tb=Tb° + △Tb
Tf=Tf° - △Tf
as concentration increases, what happens to final boiling and freezing points of solution?
increase in concentration = increase BP, decrease FP
osmosis
flow of solvent into solution through a semipermeable membrane
semipermeable membrane
solvents (ex: water) can cross, no solute molecules can pass through
water molecules move from high → low concentration until concentrations are equal
high solute concentration = low water concentration
osmotic pressure
result of increased hydrostatic pressure on the solution than on the pure solvent
how to calculate osmotic pressure
pi = iMRT
if solute is non electrolyte, assume i = 1
on formula sheet!
how to solve for vapor pressure
calculate for mole fraction (XA)
if electrolyte: XH2O= moles H2O/ moles H2O + moles of ions
plug into Raoult’s Law: Pz = XZPZ°
van’t hoff factor (i)
number of particles in solution when 1 mole of substance dissolves in water
accounts for increase in concentration when electrolyte dissociates
i = moles of particles in solution / moles of solute dissolved
if solute is strong electrolyte, I is included as multiplier (ex: iKbm = △Tb)