Exam 2: Serotonin and Ach
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
69 Terms
what kind of molecule is 5 HT?
indolamine (core structure is indole with amine sidechain)
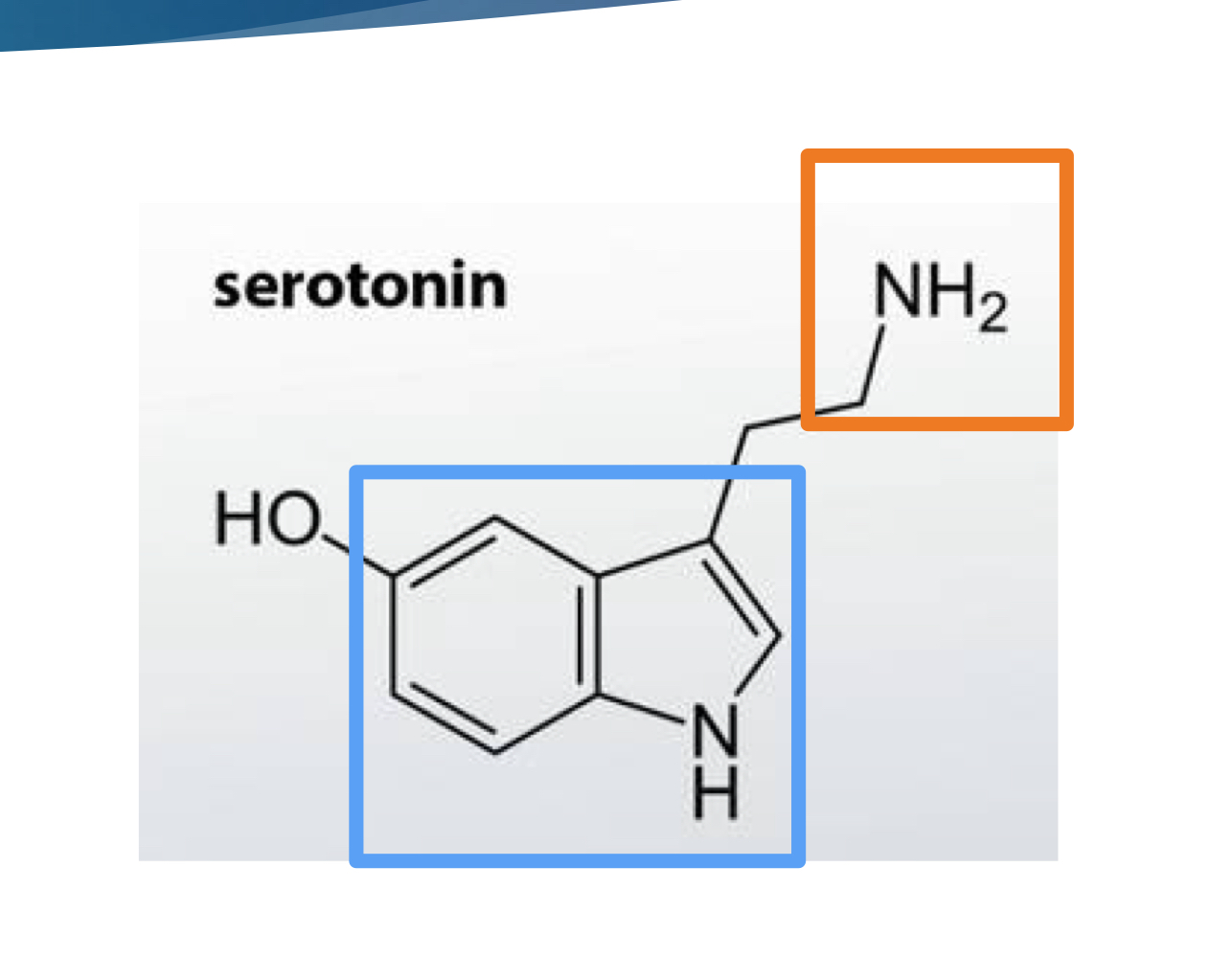
T or F: 5 HT only serves as a neurotransmitter
F: serves as an NT, neuromodulator, and hormone
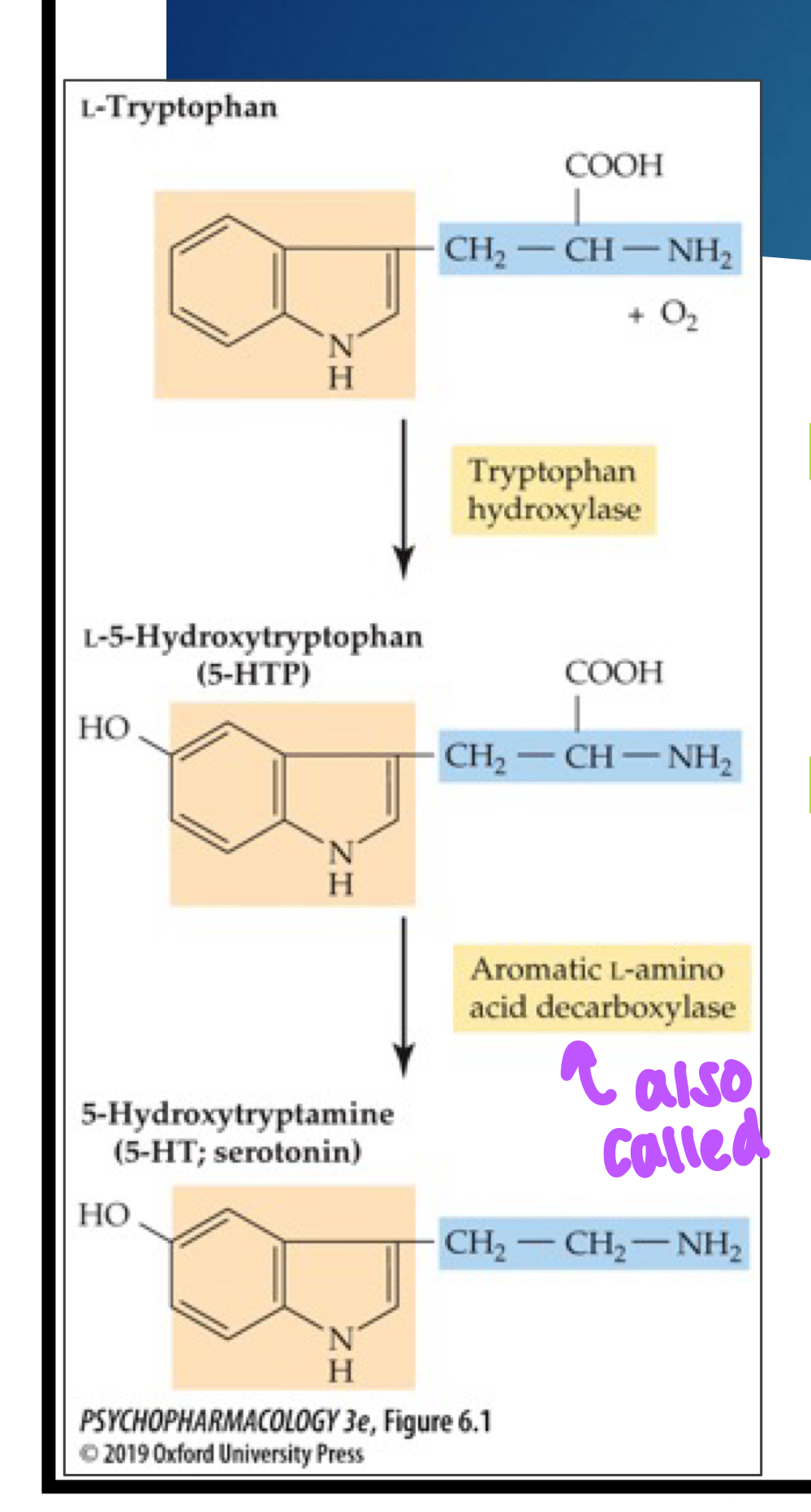
synthesis of 5HT
Tryptophan is converted to 5-HTP by tryptophan hydroxylase (TPH)
5-HTP is converted to 5-HT by AADC (AKA tryptophan decarboxylase) enzyme
difference in location of TPH enzyme subtypes
TPH1: converts tryptophan to 5-HTP (later converted to 5-HT) in non-neuronal cells located in gut and pineal gland
TPH 2: does same function in neuronal cells
which enzyme is a better indicator of 5-HT presence: TPH or AADC?
TPH
what is the rate-limiting enzyme in 5-HT synthesis?
TPH; conversion of tryptophan to 5-HTP occurs at slower rate than the conversion of 5-HTP to 5-HT
how can TPH enzyme activity be sped up?
via phosphorylation by protein kinases
where is the majority of 5-HT found in the body?
in the gut (not the brain!)
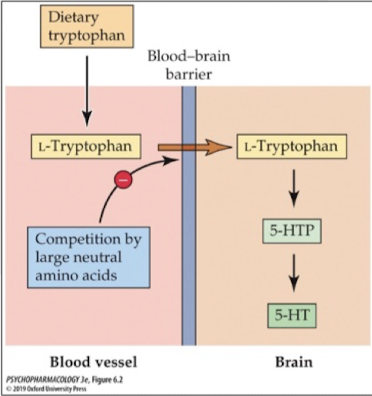
rat study of food intake and 5-HT synthesis: high protein low carbs
found that feeding rats more protein-rich foods (high in tryptophan) leads to increases in blood tryptophan but not necessarily brain tryptophan (to be converted to 5-HT)
suggests that tryptophan has to compete with other amino acids to get across the blood brain barrier
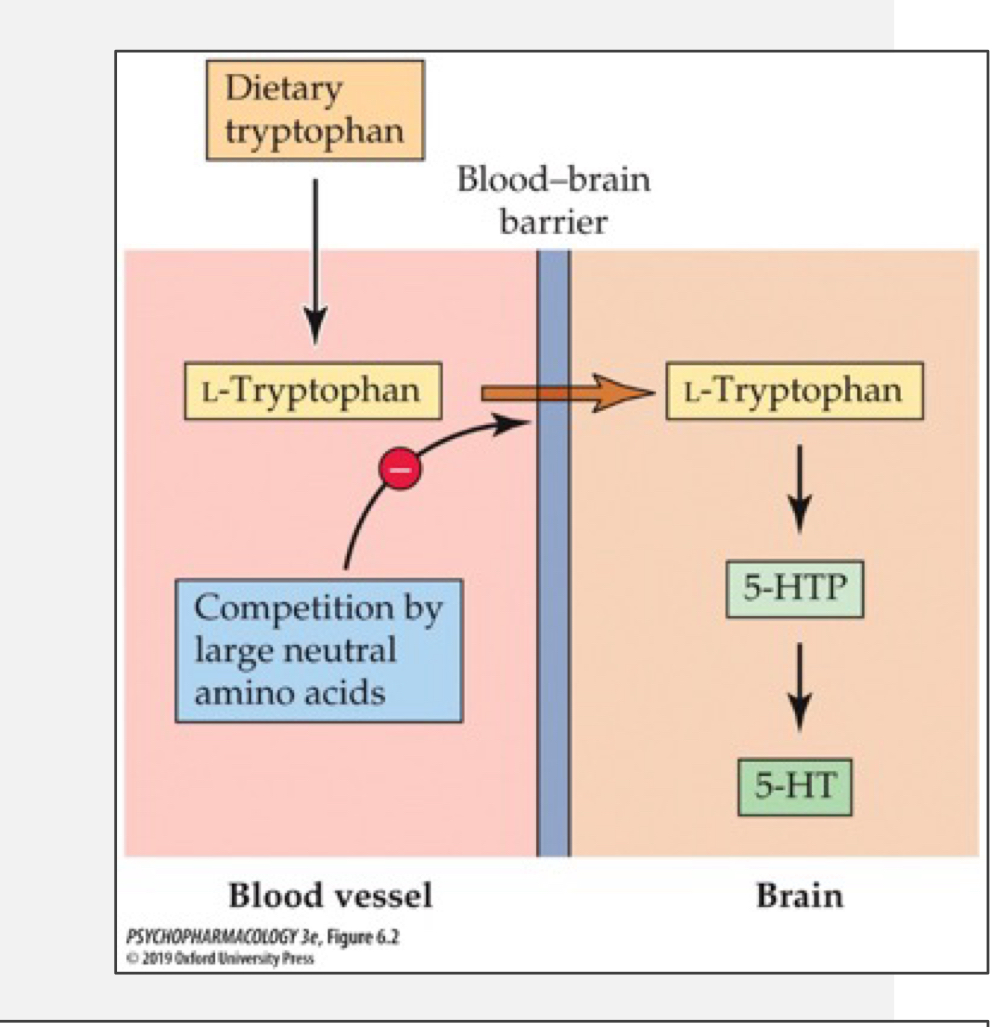
rat study of food intake and 5-HT synthesis: high carbs low protein
surprisingly found an increase in brain tryptophan
carbs cause a release. of insulin from the pancreas; insulin stimulates uptake. of glucose and many amino acids (not tryptophan) which reduces competition at the BBB allowing more tryptophan to cross and make 5-HT
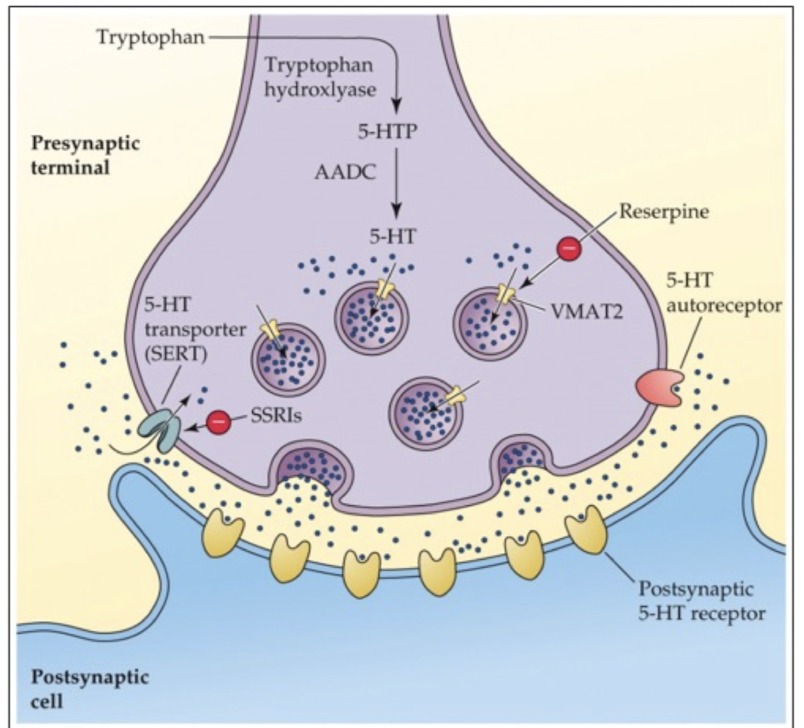
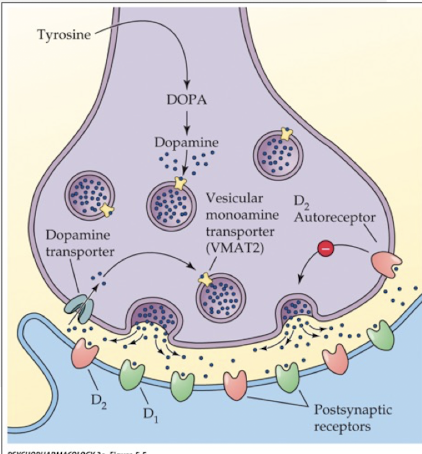
indolamine storage and release
stored in synaptic vesicles using vesicular monoamine transporters (VMAT); same as storage and release of catecholamines
where is VMAT1 located?
adrenal medulla
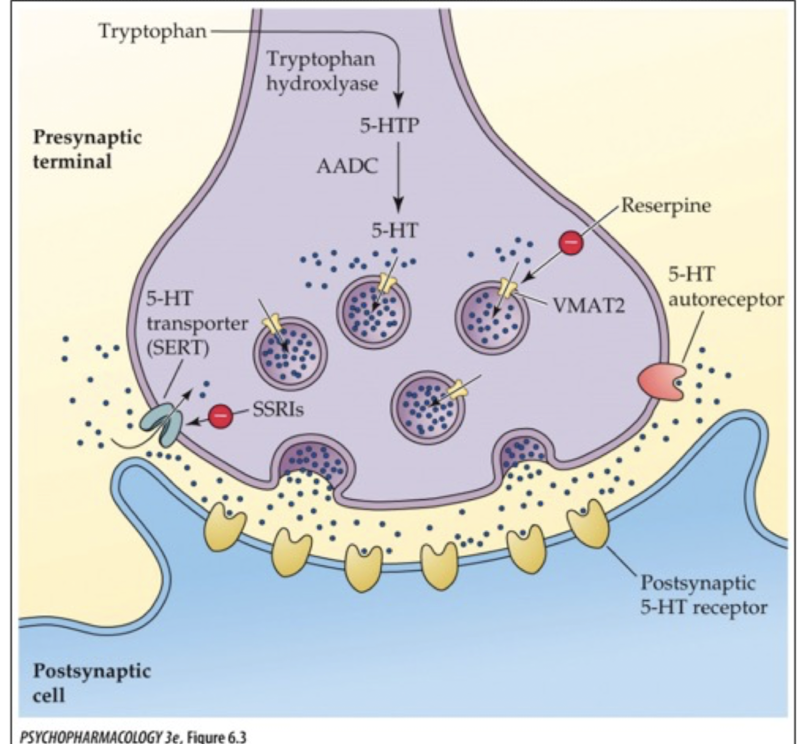
where is VMAT2 located?
CNS
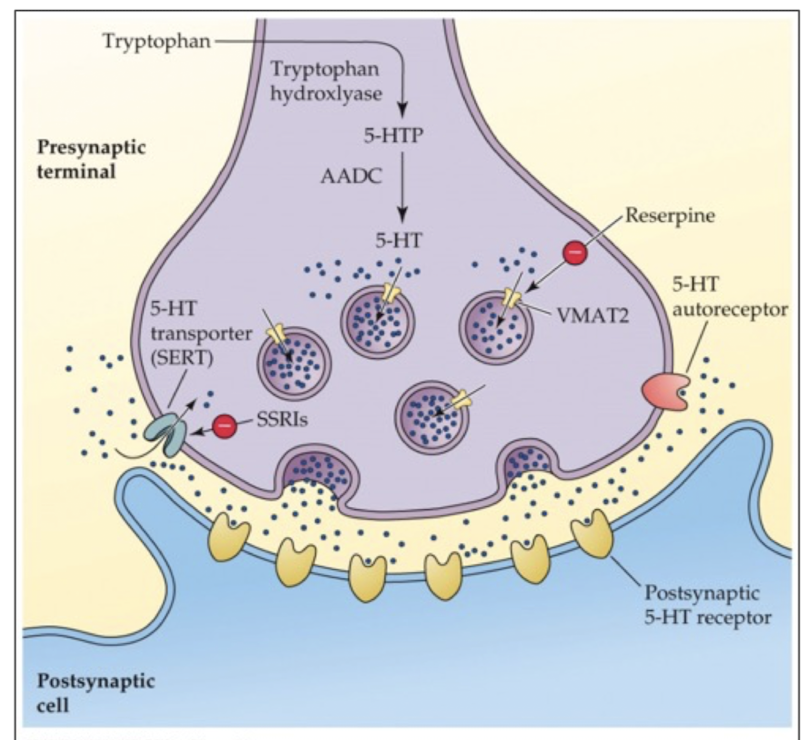
neurons release a _____ amount of NT
predetermined
purpose of VMATs
protects NT from degradation by enzymes
how is 5-HT released?
via exocytosis when the nerve terminal is stimulated
T or F: 5-HT can be released independently from nerve cell firing
true; MDMA/molly/ecstasy can cause this
how is release of 5-HT inhibited?
via autoreceptors
terminal receptors: reduce amount of Ca2+ that enters the terminal and increases opening of K+ channels causing hyperpolarization
somatodendritic autoreceptors: reduce cell firing rate
5-HT somatodendritic autoreceptor
5-HT1A receptor subtype
5-HT terminal autoreceptor
5-HT1B or 5-HT1D receptor subtype
how are indolamines inactivated?
reuptake into nerve terminal through SERT; then NT is either repackaged or metabolized to prevent excessive NT accumulation in the synapse
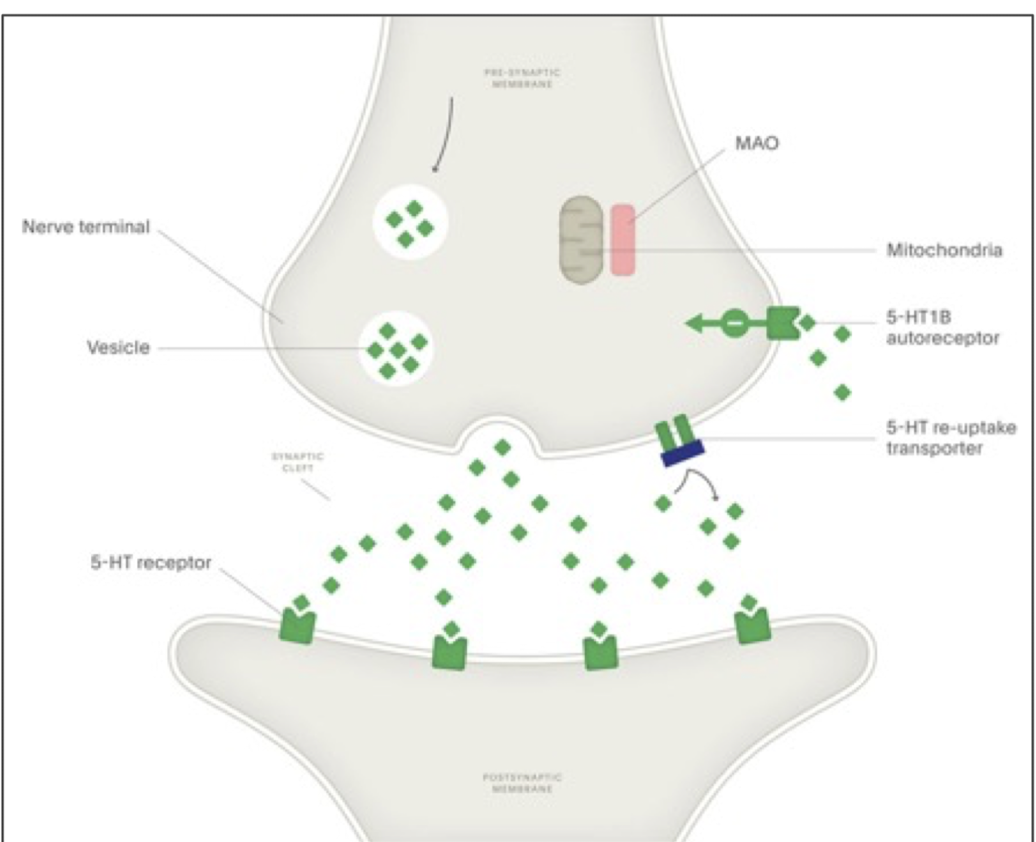
what enzyme metabolizes 5-HT
monoamine oxidase enzyme (MAO-A)
what metabolite is measured for 5-HT presence? concentration 5-HT
5-HIAA
concentration 5-HT in body (increasing to decreasing)
CSF→bloodstream→urine
where are cell bodies of 5-HT neurons in the brain?
raphe nuclei
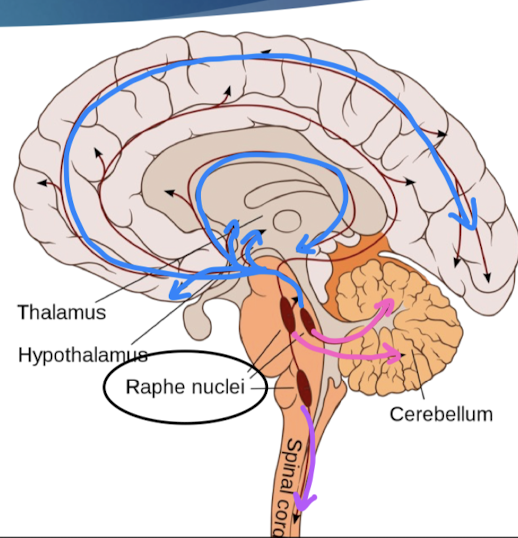
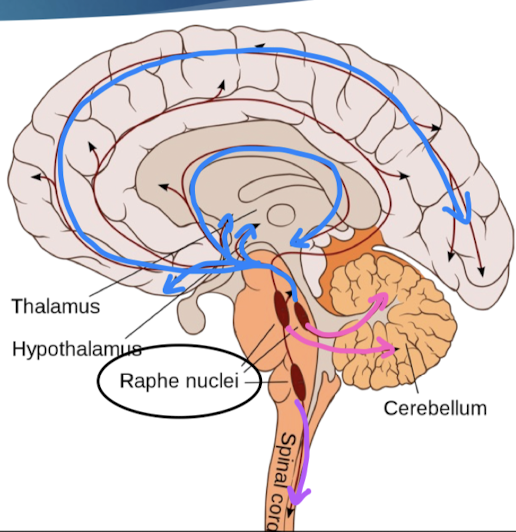
where do 5-HT neurons project?
all forebrain regions
dysfunction: mood, social behavior, appetite/digestion, sleep, memory, sexual behaviors
cerebellum
dysfunction: motor learning
spinal cord
sensory, motor, autonomic functions
how do 5-HT neurons act during wakefulness?
each cell produces tonic firing (slow but regular)
seratonin important for wakefulness!
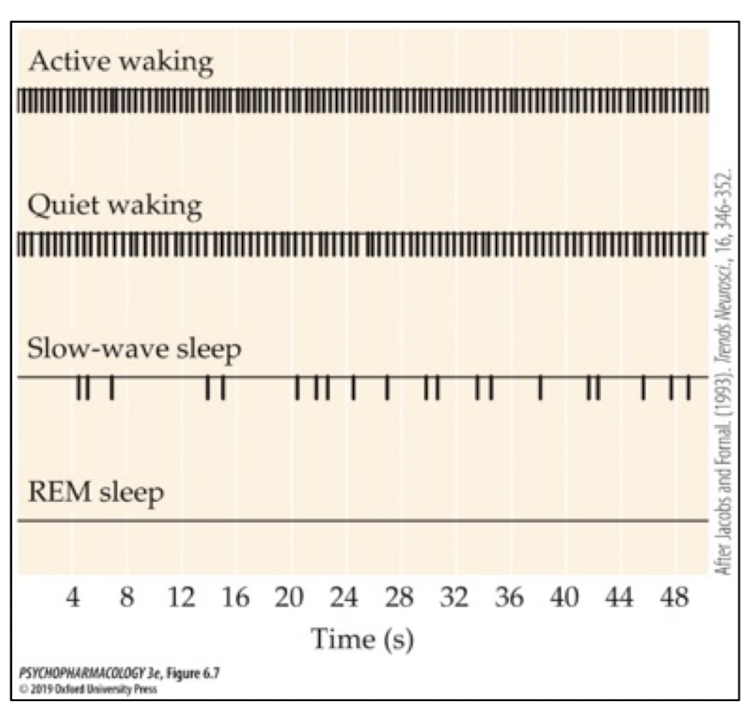
how do 5-HT neurons act during slow-wave sleep?
cell firing slows down from tonic firing (triggered by GABA) and becomes more irregular
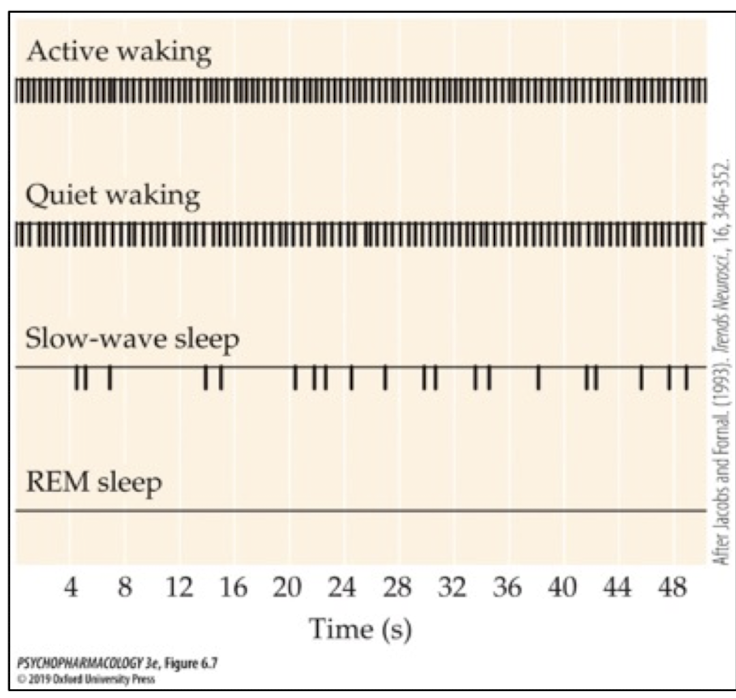
how do 5-HT neurons act during REM sleep?
firing almost completely stops

what is a phasic burst of activity?
burst of firing triggered by glutamate or ACh
facilitates motor output in cats and suppresses sensory processing
in rodents, there are phasic bursts in relation to rewards and punishments
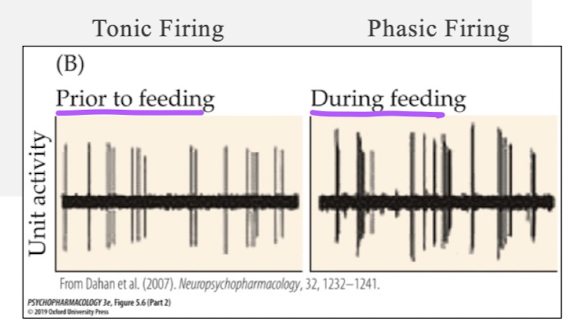
what nuclei are important in the sleep-wake cycle and release 5-HT?
raphe nuclei
total number of 5-HT receptor types?
14
metabotropic receptors for 5-HT to know
5-HT 1A, 5-HT 2A, 5-HT 5B
what happens when 5-HT binds to 5-HT1A receptor? where are these receptors found?
adenylyl cyclase is inhibited, decreases cAMP (PKA); these receptors can be autoreceptors or postsynaptic receptors
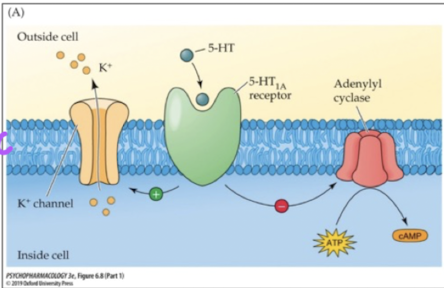
what happens when 5-HT binds to 5-HT2A receptor? where are these receptors found?
activation of phospholipase C which increases PKC
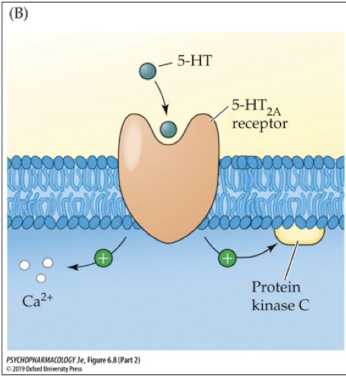
where are 5-HT2A receptors found?
cerebral cortex, striatum, nucleus accumbens
which receptor subtype do hallucinogenic drugs bind to?
5-HT 2A
5-HT1B/1D receptor
agonists; migraine medication
5-HT3 receptor
antagonists; counteract nausea and vomiting associated with chemotherapy treatment
how is aggressive behavior tested in rats?
resident-intruder paradigm; introduce an unfamiliar male into another male’s home and observe the behavior of the resident
role of 5-HT in aggressive behavior
5-HT regulates aggressive behavior, specifically impulsive aggression; when 5-HT decreases mice are more aggressive, impulsive and compulsive
5-HT is important for what behaviors?
sleep
aggression
hunger/eating
anxiety
pain signaling
learning and memory
5-HT and hunger/eating
some 5-HT receptors when activated produce hypophagia (reduced food intake) and weight loss in rodents; led to obesity medications being produced that worked on these receptors; problem was that these medications also bound to 5-HT receptors in the heart and caused heart abnormalities
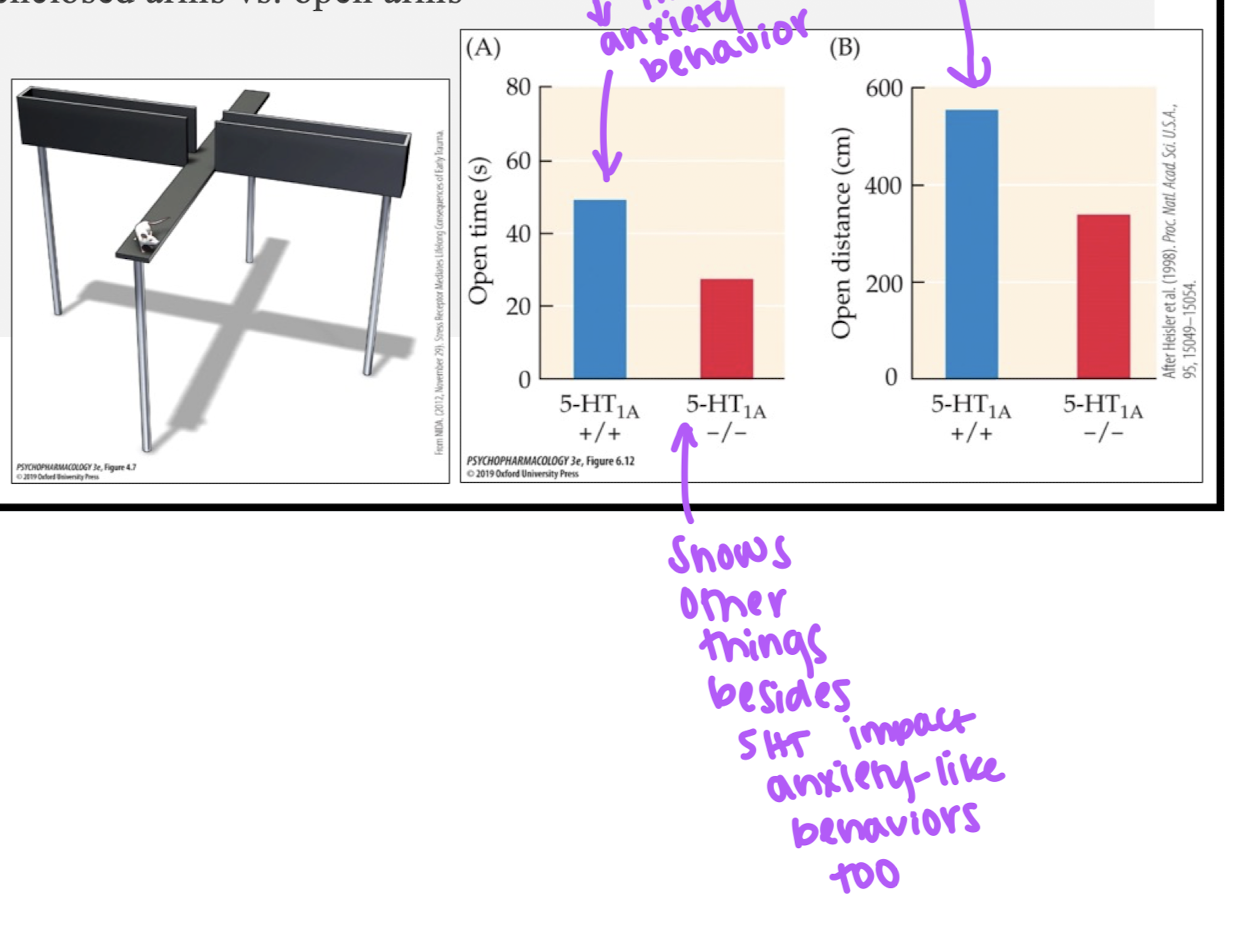
5-HT and anxiety
tested using elevated plus maze
SSRIs (5-HT1A agonists) used to help treat anxiety disorders
5-HT generally _________ pain signaling
inhibits
5-HT1A, 5-HT4, and 5-HT6 have shown promise for improving ______ performance
learning and memory
structure of acetyl choline
organic chemical made up of acetyl CoA and choline
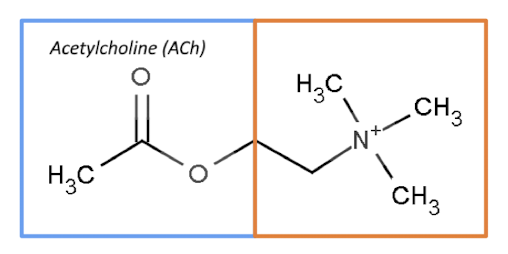
T or F: Ach acts only as an NT
False: acts as a NT, neuromodulator, and possibly an autocrine/paracrine molecule
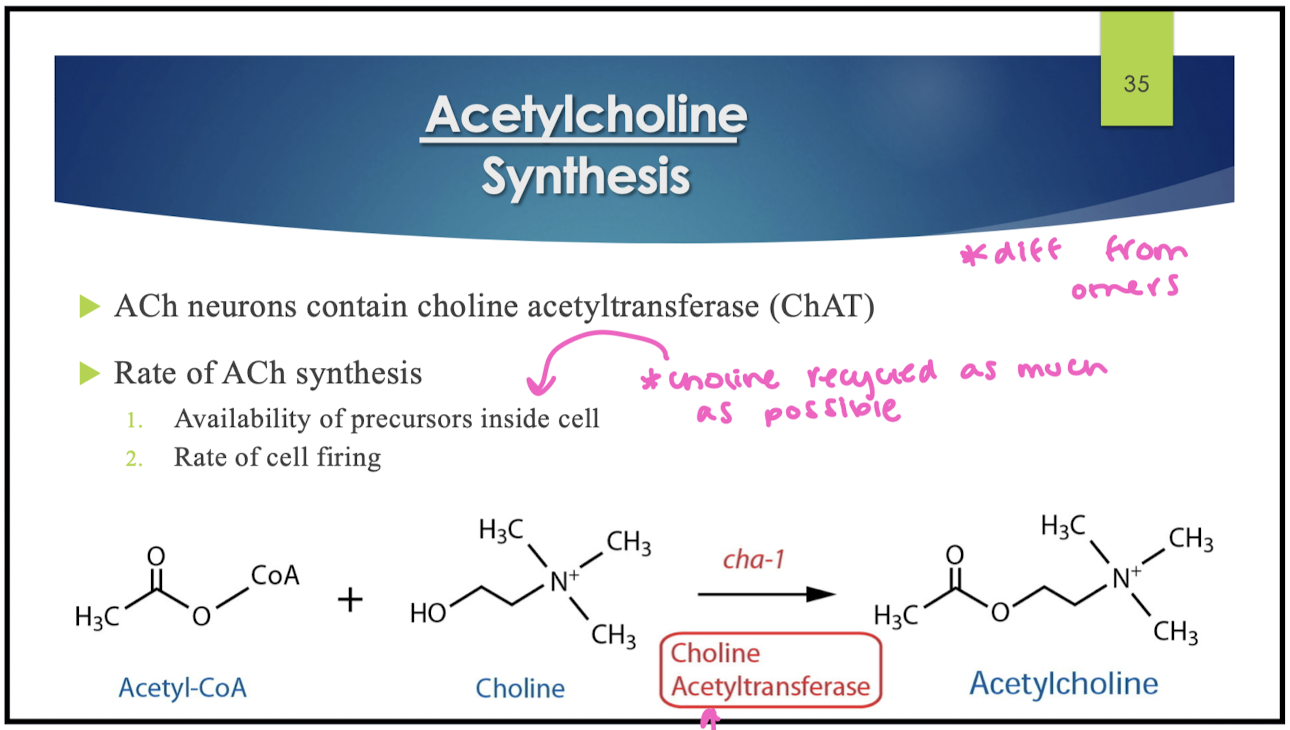
ACh synthesis
Ach neurons contain choline acetyltransferase (ChAT) that brings acetyl-CoA and choline together to make Ach
rate of ACh synthesis is based on 2 things:
availability of precursors inside the cell
rate of cell firing
ACh storage
stored in synaptic vesicles using vesicular cholinergic transporters (VAChT)
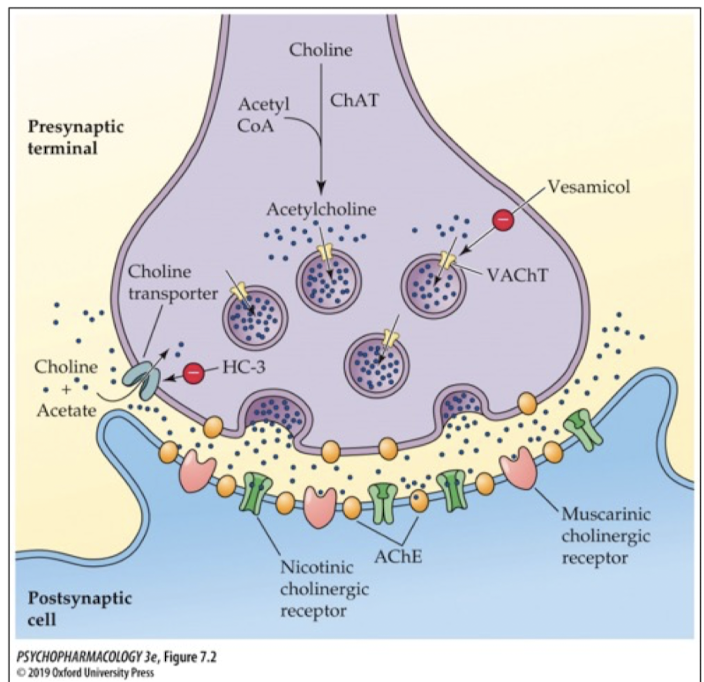
ACh release
released via exocytosis when terminal is stimulated; neurons release a predetermined amount of NT
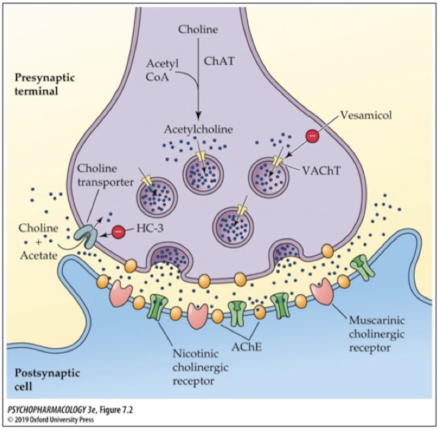
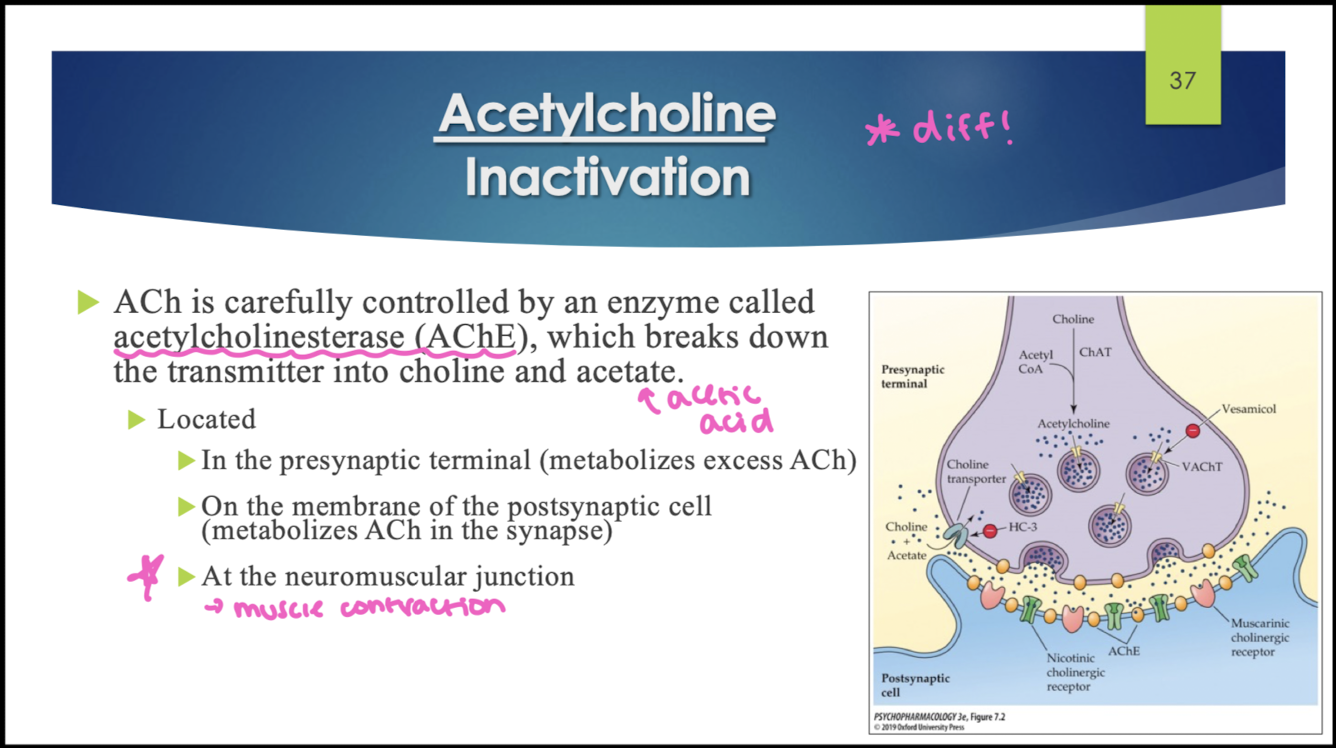
what enzyme breaks down ACh to inactivate it?
acetylcholinetransferase (AChE); breaks down ACh into choline and acetate (acetic acid)
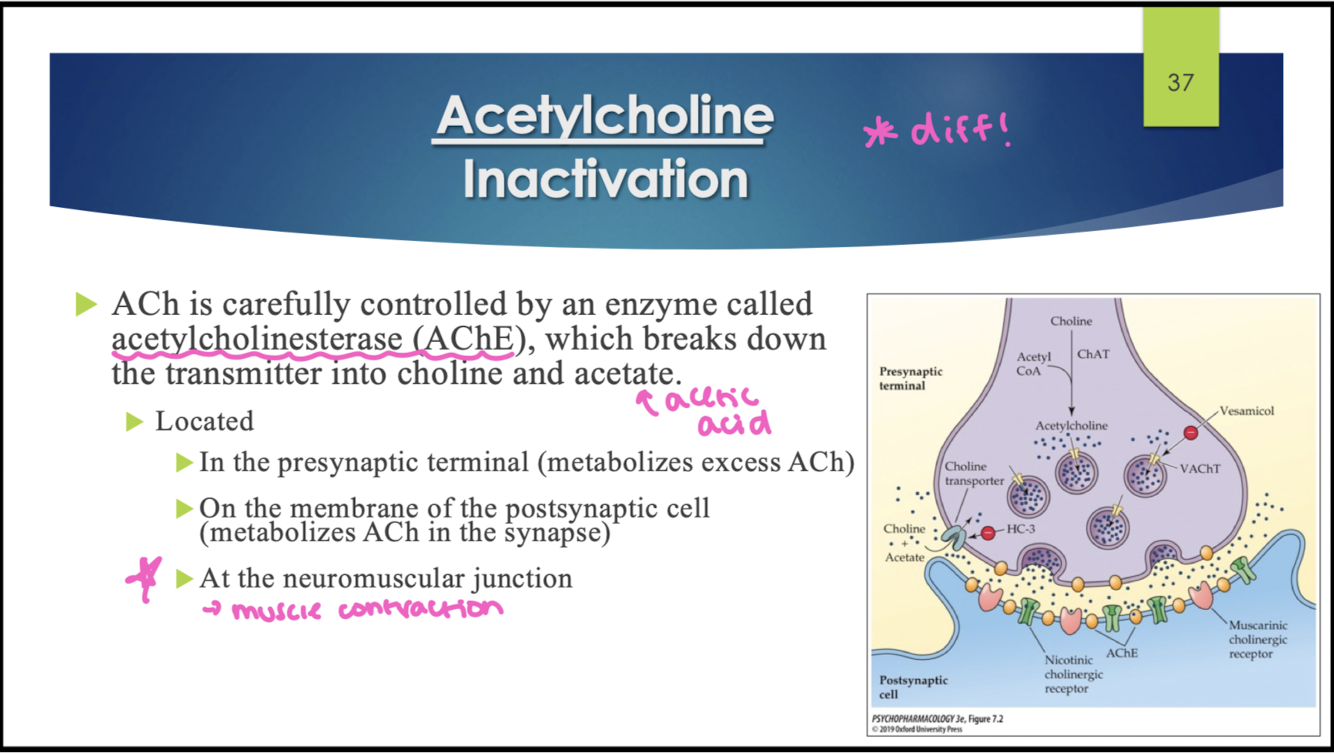
where is AChE enzyme found?
in the presynaptic terminal (metabolizes excess ACh)
on. themembrane of the postsynaptic cell (metabolizes ACh in the synapse)
At the NMJ
AChE at the neuromuscular junction
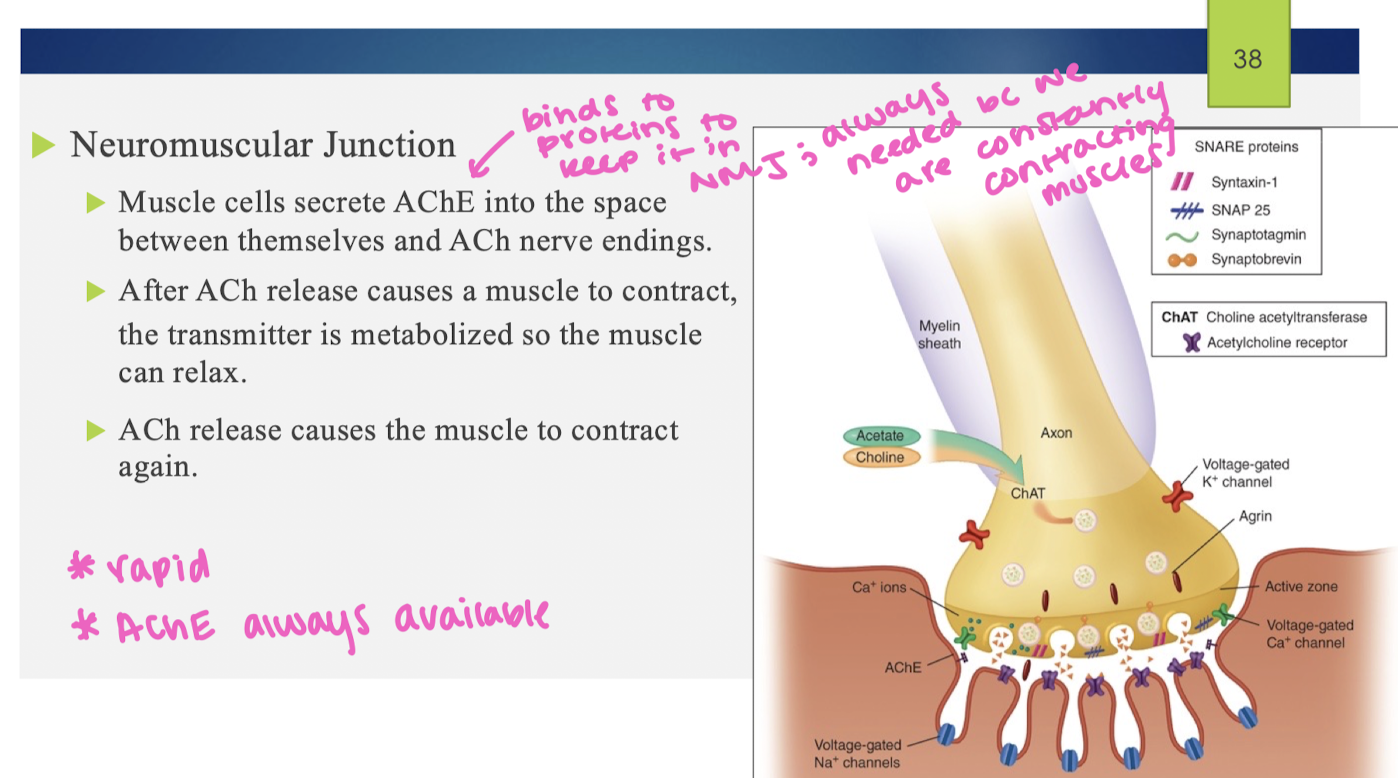
ACh reuptake
once ACh is broken down into choline, choline is taken back into the nerve terminal via a choline transporter; blocking the transporter with an antagonist leads to a decrease in ACh production (choline is a necessary precursor that must get recycled back into the cell to make ACh)
How can AChE inhibitors be used to treat diseases like Alz?
these inhibitors decrease the breakdown of ACh to increase the amount of ACh able to bind to the decreasing amount of cholinergic receptors (cholinergic neurons are dying)
_________ produce a nearly irreversible inhibition of AChE activity
organophosphorus (found in insecticides and nerve gases used in biological warfare); causes overexcited ACh receptors and symptoms like runny nose, crying, drooling, blurry vision, and eventually convulsions/death
treatments for organophosphate poisoning
atropine; competes with ACh for receptor binding (competitive, reversible antagonist); injected IV
pyridostigmine bromide (PB); competes with organophosphate for binding to AChE; acts as a reversible inhibitor of the enzyme
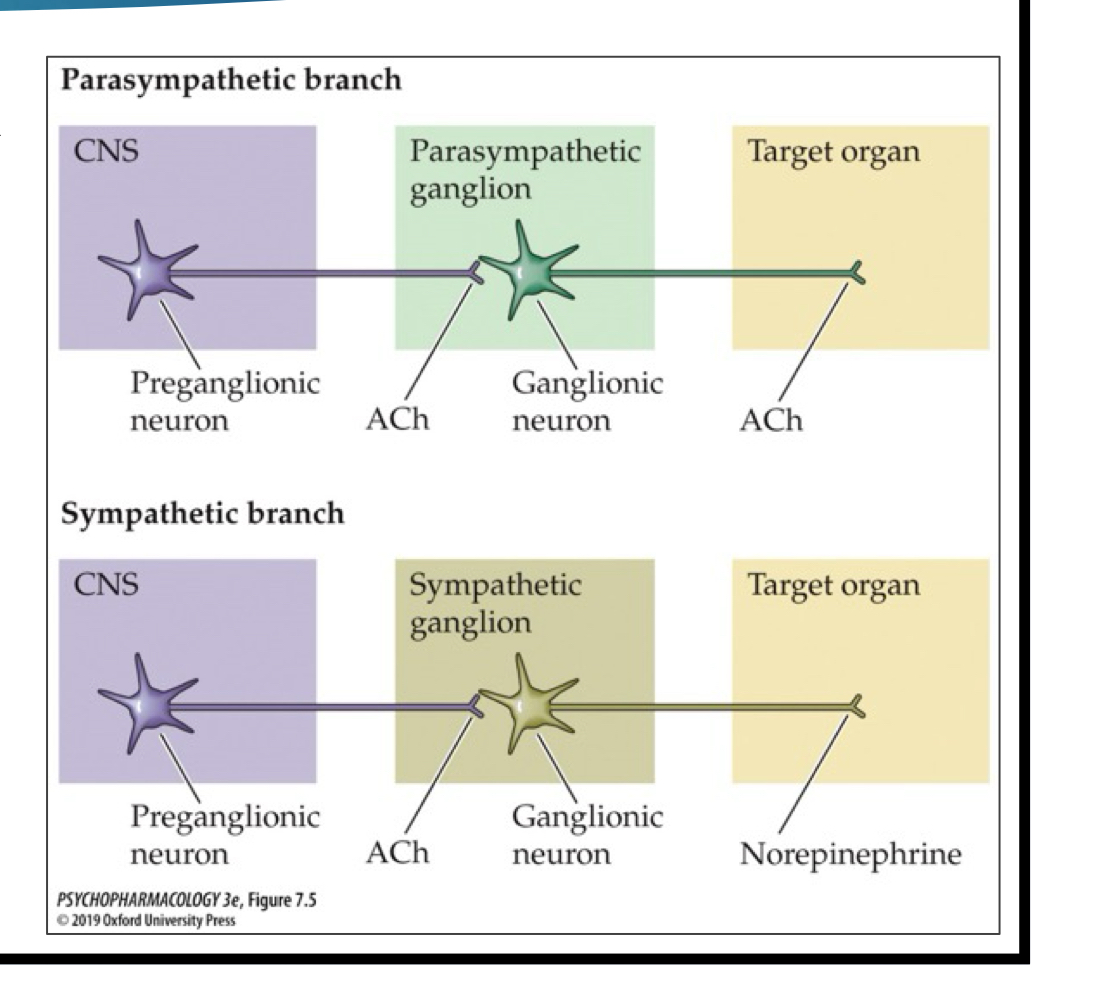
ACh pathways: parasympathetic
only ACh NT being released in the pathway
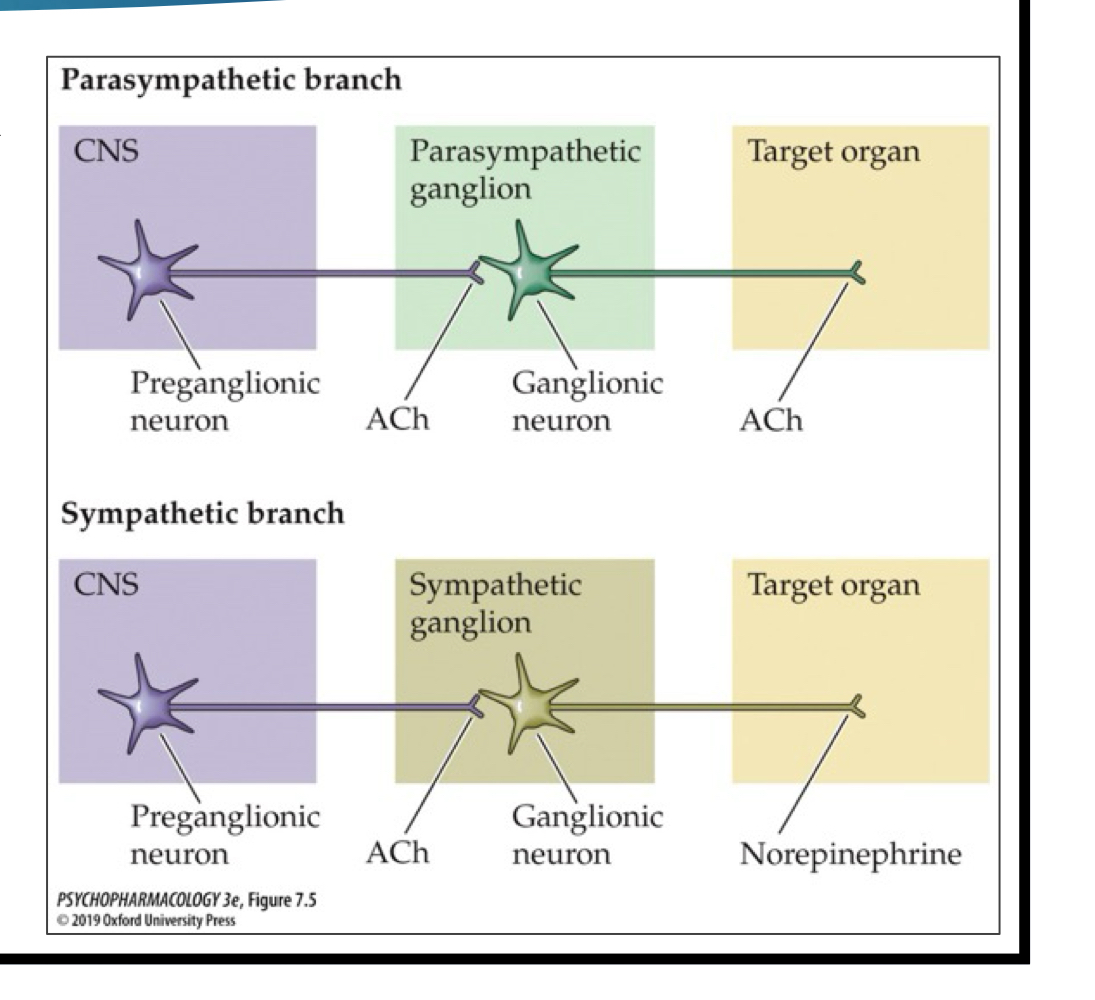
ACh pathways: sympathetic
ACh is released brom presynaptic neuron onto sympathetic ganglion neuron; NE is released onto target organ
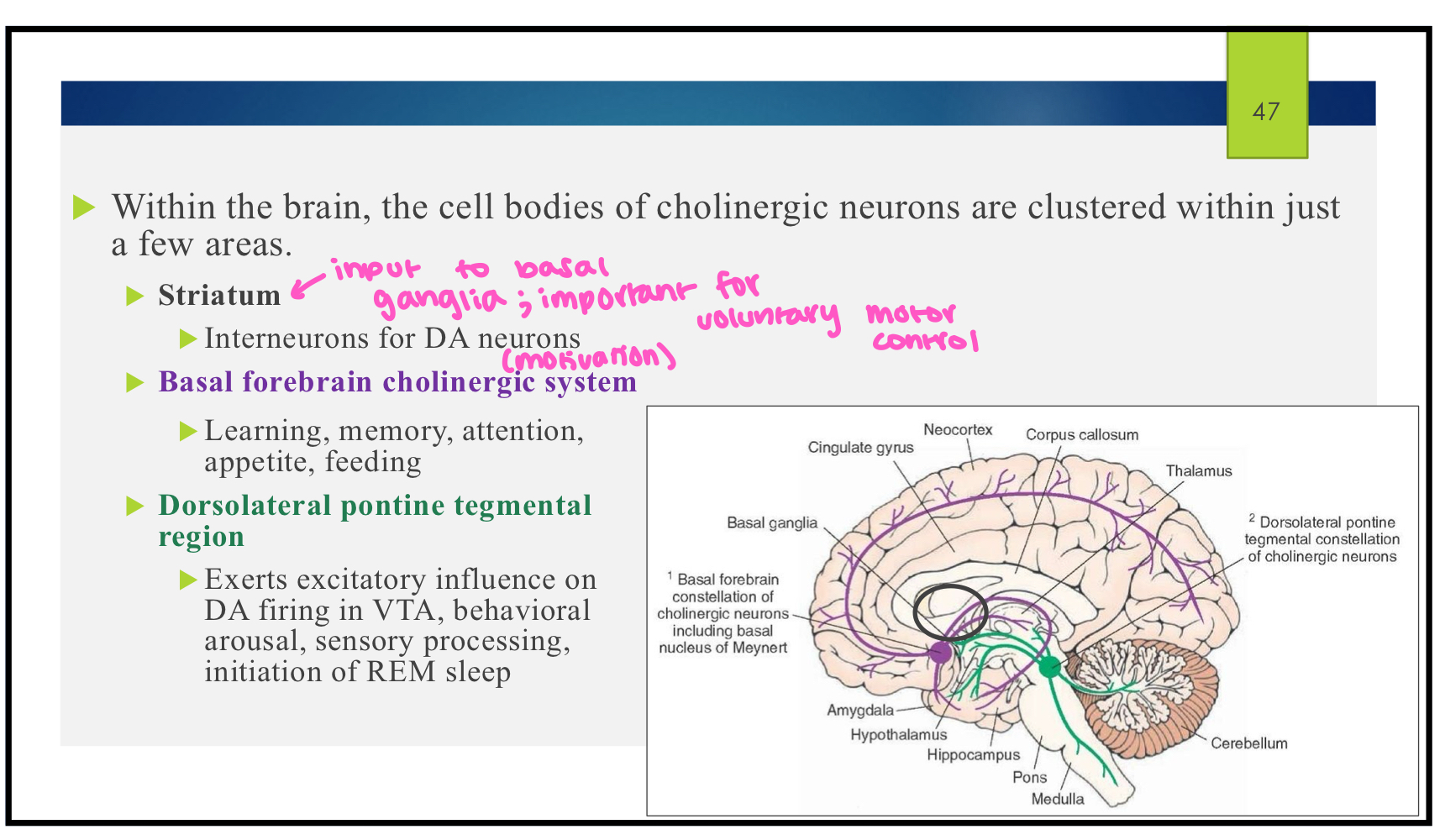
where are the cell bodies of cholinergic neurons?
striatum
interneurons for DA neurons
basal forebrain cholinergic system
learning, memory, attention, appetite, feeding
dorsolateral pontine tegmental region
exerts excitatory influence on DA firing in VTA, behavioral arousal, sensory processing, initiation of REM sleep
two types of ACh receptors
nicotinic (ionotropic); respond to nicotine
muscarinic (metabotropic); respond to muscarine
what type of receptors are nicotinic receptors
ionotropic
where are nicotinic receptors located?
muscle cells (NMJ), ganglionic neurons of both parasympathetic and sympathetic NS, neurons
PNS neurons: mostly postsynaptic (regulate cell firing)
CNS neurons: mostly presynaptic (regulate NT release)
how many subunits make up the nicotinic receptor?
5 (a, B, gamma, delta, epsilon); subunit composition differs between PNS vs. CNS neurons and muscle cell receptors
how many ACh must bind to a nicotinic receptor in a muscle cell?
2
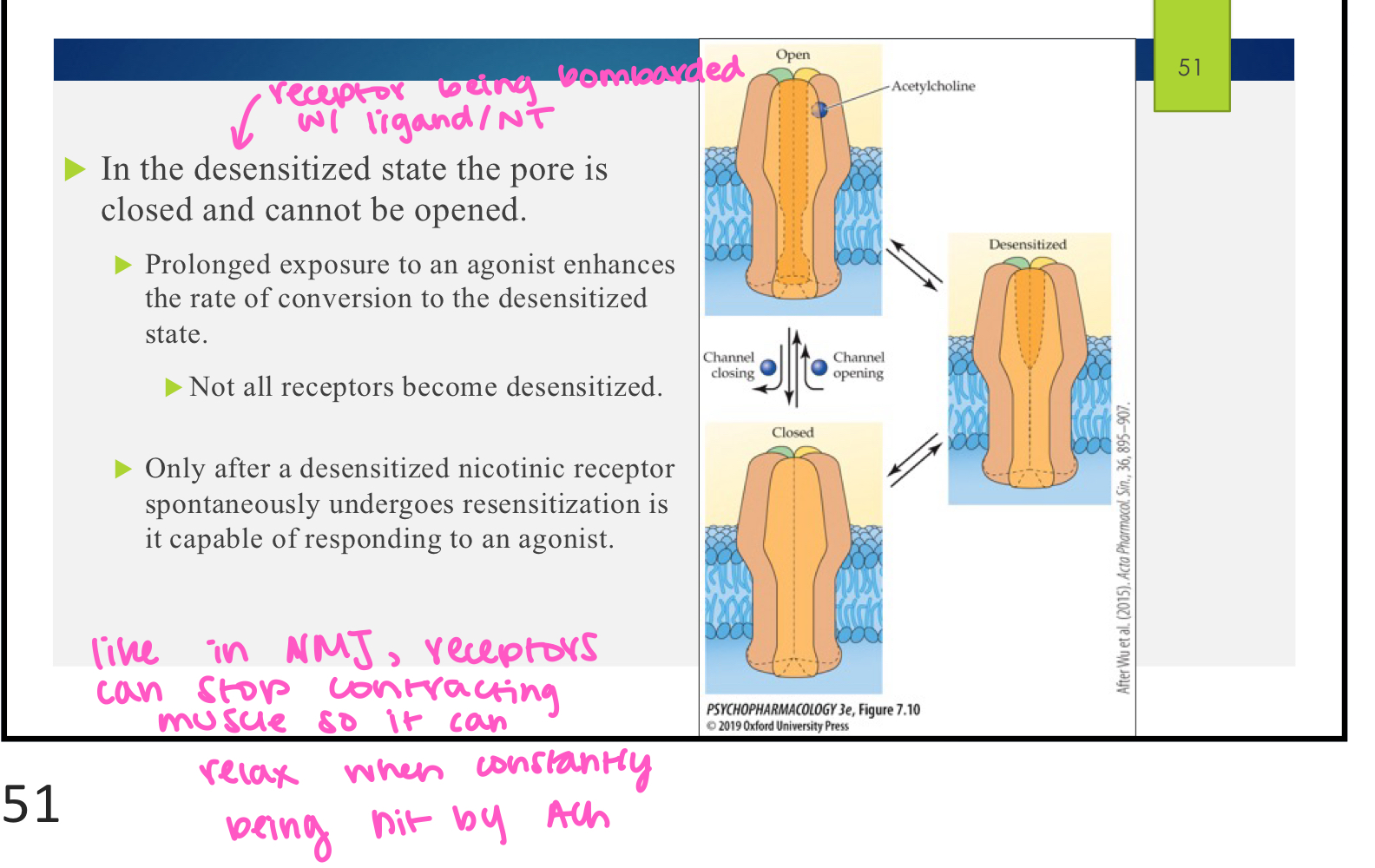
T or F: nicotinic receptors have a desensitized state
true these receptors can enter a desensitized state when there is a lot of ACh in the synapse; this causes the channel to close on the receptor and it is unable to be opened even in presence of agonist
where are muscarinic receptors found?
CNS: neocortex, hippocampus (memory), thalamus, striatum (motor), basal forebrain
PNS: cardiac muscles, smooth muscles, of organs (bronchioles, stomach, intestines, bladder, urogenital organs)
what are the subtypes of the muscarinic receptor? are they excitatory or inhibitory?
M1-M5
M1, M3, M5: excitatory
M2, M4: inhibitory