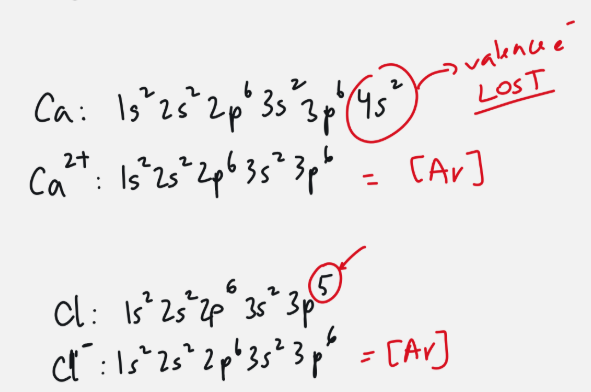electron configuration
1/9
Earn XP
Description and Tags
page 3-5 25/7/25 note;
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
electron configuration is
electron arrangement in orbitals
energy level diagram
visual representation of levels
s: one orbital
p: three orbitals, px, py, pz
d: five orbitals
f: ten orbitals
s starts at 1
p starts at 2
d starts at 3
f starts at 4
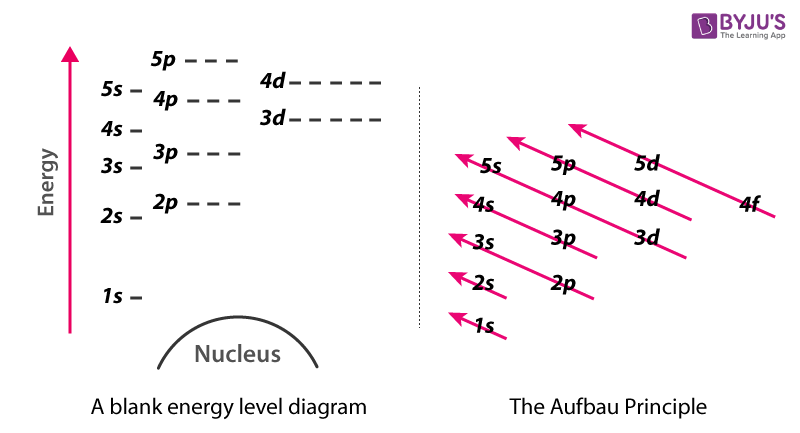
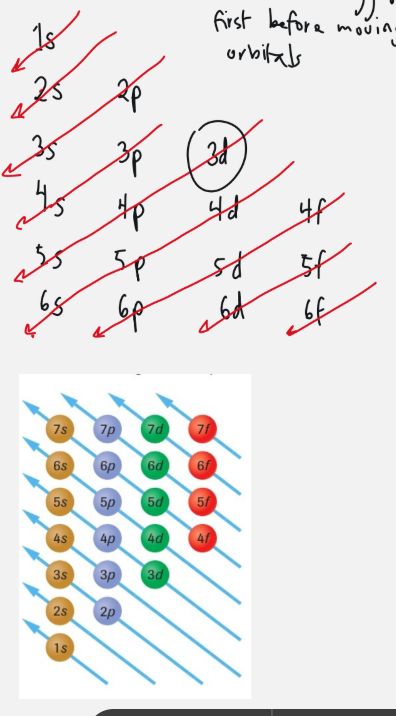
aufbau’s principle
fill lower energy orbitals with electrons first before moving up to higher energy orbitals
Hund’s rule
every orbital must be occupied by one electron first, before the electrons are paired up
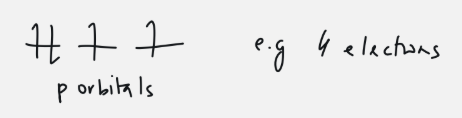
Pauli’s exclusion principle
no electron can have the same four quantum numbers, atleast one WILL differ, double check work if not
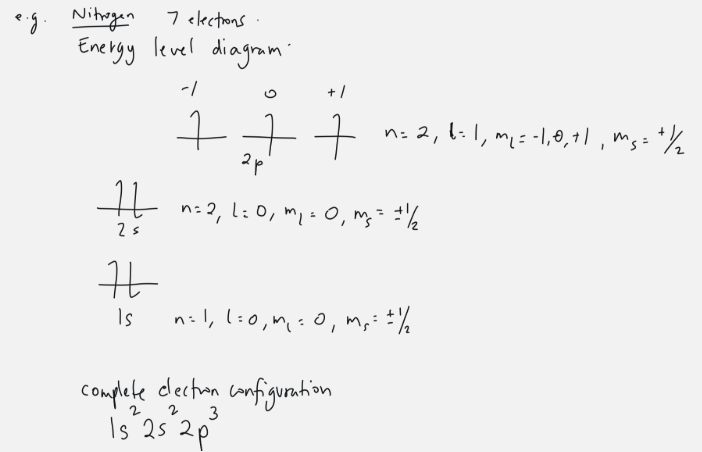
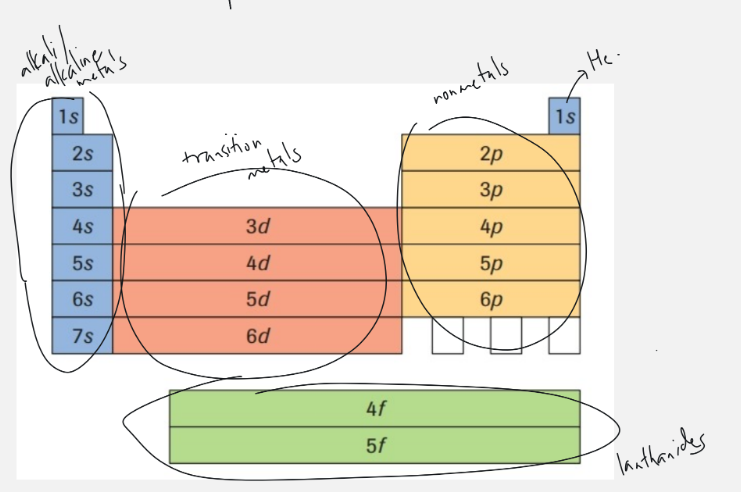
quantum number of families on periodic table
top to bottom
alkali and alkaline earth metals, including helium: 1s, 2s, 3s, 4s, 5s, 6s, 7s
transition metals: 3d, 4d, 5d, 6d
nonmetals: 2p, 3p, 4p, 5p, 6p
lanthanides: 4f, 5f
complete electron configuration
will go in order of Aufbau’s principle and end at quantum number of associated spot on periodic table
exponent indicates position on period (row)
total sum of exponents indicates number of electrons and
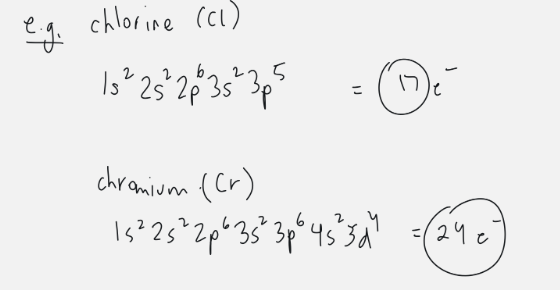
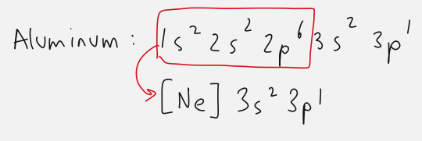
condensed electron configuration
start with noble gas before element and add rest of orbitals
special stability
when electron is promoted to next orbital
mainly with d orbitals
only with exponent 4 or 9
d orbital has one electron in four orbitals and a full s orbital → one electron from s moves to empty d
d orbital has four full orbitals, one half full, and a full s orbital → one electron from s moves to last d orbital
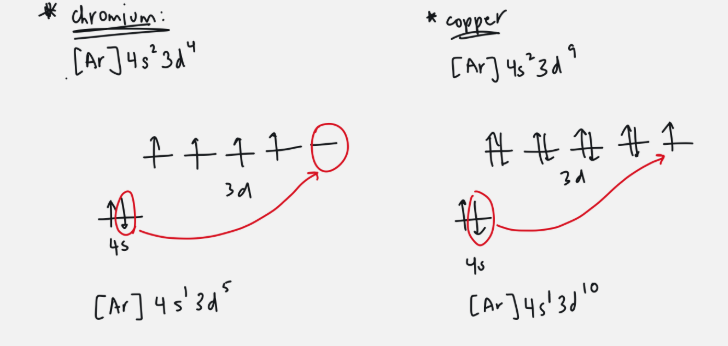
electron configuration for ions
electrons do not equal protons
add lost or gained electrons to toal
adjust in exponents
may be equal to another element