Lesson 2. States of Matter related to Pharmaceutical Formulations
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
117 Terms
Intermolecular Forces
forces of attraction and repulsion between atoms and molecules
Cohesive Forces
like molecules are attracted to each other such as beaker with water
Adhesive Forces
Different molecules are attracted in water such as pipette in water
Repulsive Forces
like poles will repel while unlike poles attract
gradual and negative (attractive)
Lennard-Jones Potential:
as two molecules approach each other at a moderate distance, the energy changes are _________________ to a point where a minimum in the potential energy occurs
attractive forces
Low/Negative Potential Energy = __________________ = needs minimum energy
repulsive forces
At a close distance, the energy starts rising rapidly as the intermolecular distances become smaller and ________________ begin to dominate
repulsive forces
positive potential energy = ________________ = needs more energy
repulsion
Moving the molecules closer results in electron cloud a.__________, whereas separating the molecules apart increases the b.__________
a = ?
attraction
Moving the molecules closer results in electron cloud a.__________, whereas separating the molecules apart increases the b.__________
b = ?
1 – 7
Intermolecular Attractive Forces — Van der Waals Forces
dipole-dipole (Keesom forces)
Energy (kcal/mole): ?
Examples:
Applications:
H2O, HCl, Alcohol, Acetone, Phenol
Intermolecular Attractive Forces — Van der Waals Forces
dipole-dipole (Keesom forces)
Energy (kcal/mole):
Examples: ?
Applications:
stabilize protein secondary structure (α–helices)
Intermolecular Attractive Forces — Van der Waals Forces
dipole-dipole (Keesom forces)
Energy (kcal/mole):
Examples:
Applications: ?
1-3
Intermolecular Attractive Forces — Van der Waals Forces
dipole-induced dipole (Debye forces)
Energy (kcal/mole): ?
Examples:
Applications:
ethyl acetate, methylene chloride, ether
Intermolecular Attractive Forces — Van der Waals Forces
dipole-induced dipole (Debye forces)
Energy (kcal/mole):
Examples: ?
Applications:
stabilizing effect on states of matter
Intermolecular Attractive Forces — Van der Waals Forces
dipole-induced dipole (Debye forces)
Energy (kcal/mole):
Examples:
Applications: ?
0.5 - 1
Intermolecular Attractive Forces — Van der Waals Forces
induced dipole-induced dipole (London forces)
Energy (kcal/mole): ?
Examples:
Applications:
Carbon disulfide, Carbon tetrachloride, hexane
Intermolecular Attractive Forces — Van der Waals Forces
induced dipole-induced dipole (London forces)
Energy (kcal/mole):
Examples: ?
Applications:
liquefaction of gases; molecular interactions in solubility, complexation, other physical bonding phenomena
Intermolecular Attractive Forces — Van der Waals Forces
induced dipole-induced dipole (London forces)
Energy (kcal/mole):
Examples:
Applications: ?
1 – 7
Intermolecular Attractive Forces — Ion-dipole Forces
ion-dipole
Energy (kcal/mole): ?
Examples:
Applications:
quaternary ammonium ion with tertiary amine
Intermolecular Attractive Forces — Ion-dipole Forces
ion-dipole
Energy (kcal/mole):
Examples: ?
Applications:
crystalline pharmaceutical salts
Intermolecular Attractive Forces — Ion-dipole Forces
ion-dipole
Energy (kcal/mole):
Examples:
Applications: ?
none
Intermolecular Attractive Forces — Ion-dipole Forces
ion-induced dipole
Energy (kcal/mole): ?
Examples:
Applications:
Potassium Iodide and Iodine
Intermolecular Attractive Forces — Ion-dipole Forces
ion-induced dipole
Energy (kcal/mole):
Examples: ?
Applications:
solubility of ionic crystalline subs in H2O
Intermolecular Attractive Forces — Ion-dipole Forces
ion-induced dipole
Energy (kcal/mole):
Examples:
Applications: ?
Description of Hydrogen Bonding
strong type of dipole-dipole
attraction of a hydrogen atom for a strongly negative atom
Application of Hydrogen Bonding
protein α–helix and β–sheet structures
conformation of proteins, physical properties of alcohols compared to alkanes
carboxylic acids compared to esters, aldehydes, and ketones
sugars
Description of Hydrophobic interaction
forces of attraction between nonpolar atoms and molecules in water
Application of Hydrophobic Interaction
structure and stabilization of molecules including proteins and bilayer membrane structures
Gas
higher kinetic energy
weak intermolecular forces
Liquid
denser possesses less kinetic energy than gases
Solid
strong intermolecular forces
little kinetic energy
PV = nRT
Ideal Gas Law Equation
directly
According to the Ideal Gas Law, Pressure and Volume are ______________ proportional to the number of moles and temperature.
Ideal Gas Law
useful in calculating properties of gases at atmospheric pressure and at temperatures above their boiling points
indirectly
According to the Boyle’s Law, Volume is ___________ proportional to the Pressure
directly
According to Charles’ Law, Volume is ___________ proportional to Temperature
directly
According to Avogadro’s Law, Volume is ___________ Proportional to the number of moles.
Anesthesia, Blood Gases, and Oxygen
Application of gas in medicine
blood gases
oxygen and carbon dioxide
Henry’s Law of Gas Solubility
the amount of gas dissolved in the plasma is proportional to the partial pressure of the gas in equilibrium with the plasma
Dalton’s Law of Partial Pressures
the partial pressure is the pressure a gas would exert if it alone occupied the whole volume of the mixture
increases
vapor pressure __________ with temperature
inversely
vapor pressure and boiling point are ____________ related
boiling point
temperature in which the vapor pressure of a liquid equals the atmospheric pressure
Surface Tension
force per unit length
decreases
surface tension ____________ with an increase in temperature
Crystalline solids
molecules or atoms are arranged in repetitious three-dimensional lattice units (unit cell) infinitely throughout the crystal
Cubic
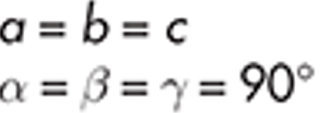
Sodium Chloride
example of cubic
Tetragonal
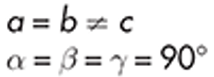
urea
example of tetragonal
Orthorhombic
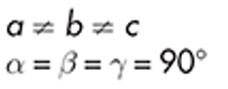
ritonavir form II
example of orthorhombic
Rhombohedral
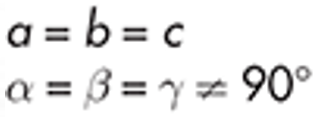
iodine
example of rhombohedral
Hexagonal
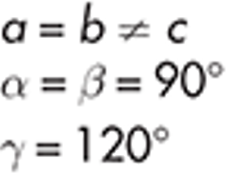
iodoform
example of hexagonal
Monoclinic
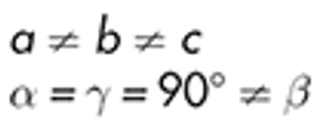
sucrose, ritonavir form I
example of monoclinic
Triclinic
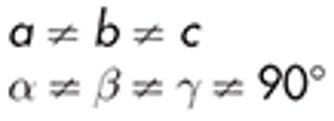
boric acid
example of triclinic
Carbon, Diamond
TYPES OF CRYSTAL BONDING:
Unit: Atom to atom
Example: ?
Bonding:
Physical Characteristics:
Strong carbon covalent bonds
TYPES OF CRYSTAL BONDING:
Unit: Atom to atom
Example:
Bonding: ?
Physical Characteristics:
Hard large crystals
TYPES OF CRYSTAL BONDING:
Unit: Atom to atom
Example:
Bonding:
Physical Characteristics: ?
Silver
TYPES OF CRYSTAL BONDING:
Unit: Metallic
Example: ?
Bonding:
Physical Characteristics:
Strong metal bond
TYPES OF CRYSTAL BONDING:
Unit: Metallic
Example:
Bonding: ?
Physical Characteristics:
positive ions in a field of freely moving electrons
TYPES OF CRYSTAL BONDING:
Unit: Metallic
Example:
Bonding:
Physical Characteristics: ?
Menthol, Paraffin
TYPES OF CRYSTAL BONDING:
Unit: Molecular
Example: ?
Bonding:
Physical Characteristics:
van der Waals forces
TYPES OF CRYSTAL BONDING:
Unit: Molecular
Example:
Bonding: ?
Physical Characteristics:
close packing, weakly held together, low melting point
TYPES OF CRYSTAL BONDING:
Unit: Molecular
Example:
Bonding:
Physical Characteristics: ?
Sodium Chloride
TYPES OF CRYSTAL BONDING:
Unit: Ionic
Example: ?
Bonding:
Physical Characteristics:
Electrostatic Ionic Bond
TYPES OF CRYSTAL BONDING:
Unit: Ionic
Example:
Bonding: ?
Physical Characteristics:
Hard, close packing, strongly held together, high melting point
TYPES OF CRYSTAL BONDING:
Unit: Ionic
Example:
Bonding:
Physical Characteristics: ?
Polymorphs
may exist in more than one crystalline structure
changes in crystalline forms: changes in intermolecular bonding patterns conformational changes in the molecule molecular orientations between neighboring molecules in solid
physical properties: melting point, solubility, stability
Hydrate
water is included in a lattice
commonly used as drug substances
multiple hydrates can exist for a drug substance
less soluble in water or aqueous mixtures than anhydrous forms
Solvate
solvent is incorporated into the lattice
not chosen as drug substances due to possible toxicity of common solvents
Salt crystals
lattice accommodating other molecules to form salts
2 ionized compounds will interact with the lattice to form a crystalline salt
drug substance
can be a weak acid or a weak base
counterion
corresponding compound in a salt
properties
melting point, stability, solubility, dissolution, bioavailability
Cocrystal
homogeneous multicomponent phase of fixed stoichiometry where the chemical entities are held together in a crystal lattice by intermolecular forces
also contain water and solvents to form cocrystalline hydrates
good option to change properties when an ionizable group is not available
Amorphous
no long-range order over many molecular units to produce a lattice or crystalline structures
do not possess melting points
less physically stable than crystalline, more soluble than crystalline materials
glasses
nonequilibrium solid form
supercooled liquids
viscous equilibrium liquid form
glass transition (Tg) temperature
temperature where an amorphous material converts from a glass to a supercooled liquid (rubbery) upon heating
Amorphous dispersion
amorphous drug is stabilized by a polymer or a combination of polymers or surfactants
increased solubility of amorphous material with physical stability closer to crystalline material
drug : polymer ratio
Polymeric Solids
large molecules formed by covalent assembly of smaller molecules (monomers) into chain or network of repeating structural units
stabilize the amorphous drug in solid state
help prevent crystallizations
used as excipients in solid, semisolid, and liquid formulations
Natural Polymers
rubber, polypeptides, cellulose
Synthetic and Semisynthetic Polymers
polyvinylchloride, polyethylene, polystyrene, polyvinyl acetate, polyactides, methylcellulose derivatives
Liquid to Gas
intermolecular forces are related to heat of vaporization and to molecular weight
↑ MW = ↑ intermolecular points of contact = ↑ intermolecular interactions [↑ HV , ↑BP, ↓VP]
Heat of vaporization
heat absorbed when 1 g or 1 mole of liquid is vaporized
Solid to Liquid
intermolecular forces are related to heat of fusion and to molecular weight
↑ MW = ↑ intermolecular forces [↑ Hf , ↑MP]
Melting point
temperature at which the solid changes into a liquid
Heat of fusion
heat required to increase the interatomic or intermolecular distance in the solid state to form the liquid state
Pliaglis Cream
Lidocaine and Tetracaine Eutectic Mixtures:
topical local analgesia for superficial dermatological procedures
Synera Patch
Lidocaine and Tetracaine Eutectic Mixtures:
local dermal analgesia for superficial venous access and superficial dermatological procedures
Emla Cream
Lidocaine and Prilocaine Eutectic Mixtures:
topical anesthetic and local analgesia
Oraqix Periodontal Gel
Lidocaine and Prilocaine Eutectic Mixtures:
local anesthetic indicated for adults who require localized anesthesia in periodontal pockets
Chemical Stability
involves the molecule degrading into other products
oxidation, hydrolysis, cyclization
interaction of drug molecules with excipients or other drug molecules in dosage forms
chemical degradation in surface solution phase and true solid state