Lecture 11: White Blood Cells (1) - Introduction and The Innate Immune System
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
White Blood Cells
Highly flexible cells → allows them to deal with different pathogens,
Even though different cells are more suited to different pathogens, cooperation between cells types is almost always needed
more that one type of ___ cells involved in clearing an infection
Red Blood Cells
Cells that are highly specialised for a single task → O2 transport
Types of WBCs
Neutrophils
Eosinophils
Basophil
Monocyte
B-lymphocyte
T-lymphocyte
Natural (T) Killer
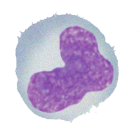
Neutrophil
String sausage nuclei
Typical granulation
‘resting form’ – circular cytoplasm
Involved in killing small organisms
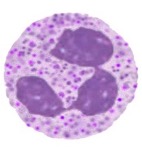
Eosinophils
Cell has an affinity for eosin dye – red colour
Multilobed, but generally 2-lobed nucleus ‘
Contain dense granules that do not overlie the nucleus
When stained appear red → acid stains
Involved in killing large organisations e.g.worms
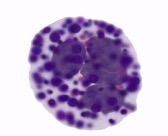
Basophils
Contains dense basophilic granules
Basophilic/ basic dyes stain granules → stained blue
Specific features in allergy response and offer protection from worms etc
Involved in killing large organisms
Resemble tissue mast cells - have a similar origin
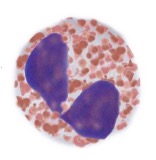
Monocytes
Helps to kill bacteria and small organisms
Helps activate B and T cells by becoming an antigen-present cell
Links the 2 systems
B-Lymphocytes
Make Antibodies
Involved in destroying toxins or blood pathogens

T-Lymphocytes
Assist B-cells in their function
Involved in fighting and killing viruses
Natural Killer Cells
Evolutionary ancient cell that recognises anything as non-self and destroys it
Involved in killing viruses
Need for So Many WBCs
To address the wide range of pathogens:
The very big: e.g. worms – 20-30x bigger than WBC, can’t ‘eat’ it to destroy
The big: e.g. bacteria – replicate rapidly
‘eaten’ by WBC due to small size
The small and hidden: e.g. viruses
Hijack cell’s enzyme machinery to produce virions that are released; spend most time in the cell – difficult to detect
The small and not hidden: e.g. toxins, initial virus exposure
(antibody removal)Available in the blood – must be detected and destroyed
Function of WBCs
Killing large organisms
Killing small organisms
Killing viruses
Destroying toxins and pathogens
Anibody response
WBCs: Killing of Large Organisms e.g. Worms
Can’t be engulfed and digested
Able to wriggle and move around, evading WBCs and going in and out of tissues
Cells need to cooperate and destroy them in the tissues while minimising the damage to normal cells
Major roles of eosinophils and basophils → immobilise the large organism so that it stays in one place to then be destroyed
muse release toxic substances
WBCs: Killing of Small Organisms e.g. Bacteria
Can be engulfed and digested safely by cells
This must be done quickly and effectively before the bacteria can cause damage
Bacteria massively outnumber the neutrophils due to rapid replication
Major roles of neutrophils and monocytes → must respond rapidly, safely and many times to ingest and remove bacteria
Neutrophils in a healthy individual in a relaxed state – must be activated to attach and ingest bacteria
It can be done by monocytes but at a slower rate – useful for slowly proliferating bacteria → can wall things in
WBCs: Killing Viruses
They infect normal cells and replicate inside them.
They are therefore hidden from the immune system.
The immune cells must therefore recognise and destroy infected cells while not affecting normal cells
Major roles of T-lymphocytes and natural killer cells → Recognition, Finding, Increase in Cell N.o, Destruction
Glandular Fever
Virus infects B-lymphocytes → a B-lymphotropic virus that invades and hides in B-cells
Activated T-cells recognise the B-cells as virally infected and briefly bind to signal it to die via apoptosis - preventing virus present inside the B-cell from being released
‘autodigests itself and any virus present inside
WBCs: Destroying Toxins or Blood Pathogens
Specific proteins (e.g. toxins, bacterial surface proteins, or free virus) may identify an invading organism or have a major effect on the body.
Spasms caused by the toxin released by bacteria (tetanus) → binds to muscle receptors and activates it
The body must recognise and respond to these small protein antigens and ideally have memory if the antigen is reencountered:
Major role of antibody-secreting B-lymphocytes
Must produce antibodies to remove the toxin itself – slow process
Role of WBCs in the Antibody Response to COVID 19
Exposure to the virus was detected by specific antibodies in the blood
The neutralising Ab was one mechanism by which immunisation was effective against the virus
Abs only present in the blood for 3-6 months after which no protection is offered
Antibodies can prevent viruses from entering cells and mark them for destruction by the immune system
T-cell response to virus is a major component to the immune response - important in providing ongoing and longer-term protection
Vaccine Design
Aims to stimulate such antibodies and are one measure of success
‘History’ of the Innate Immune System
Ancient evolutionary origin.
Present since multi-cellular origin.
Its cells appear to act in similar ways to free-living organism
Amoeba
Free living microorganism moves freely:
It recognises and ingests prey (non-self)
It recognises but does not ingest other amoeba as non-prey (self)
Why are the Cells of The Innate Immune Sytems Compared to Amoeba
Cells such as neutrophils, eosinophils, basophils and monocytes patrol blood and tissues, ignoring the normal cells (“self”) while recognising and destroying bacteria or worms (“non-self”)
Mechanism of Pathogen Recognition and Response
Neutrophils, eosinophils, basophils and monocytes recognise common proteins associated with infection.
Infected organisms have different proteins not encountered by the body (non-self)
Either the products of damage to self or the products of bacterial infection
These are called DAMPS or PAMPS and are recognised by receptors on the neutrophil surface and activate the cell so that is primed and ready to destroy
Pathogen Associated Molecular Patterns (PAMPs)
e.g. bacterial cell wall - lipopolysaccharide
Recognised as foreign
Recognised by and activates neutrophils, which then destroys/ eliminates them
Damage Associated Molecular Patterns
e.g. released bacterial components → bacterial BAM and intracellular components
Recognised as foreign
Recognised by and activates neutrophils, which then destroys/ eliminates them
Cytokines
Molecules that are released once a pathogen is recognised to help with the increase formation, (early) release and activation of white blood cells from the marrow and location tissues
E.g.
G-CSF
GM-CSF (granulocyte/monocyte colony-stimulating factor)
M-CSF(monocyte colony-stimulating factor)
together enhance the production of monocytes and granulocytes.
Prime the immune system – activate neutrophils and monocytes
G-CSF
Cytokine that enhances the production and release of neutrophils
Activation causes them to become more motile, more sticky and more likely to engulf things
It also increases neutrophil adhesion, granulation and responsiveness → increases the rate at which the proliferative compartment will mature and increases survival
increased protein synthesis
increased granular content,
activated cytoskeleton
more capable of wriggling and have increased phagocytic abilities → more likely to attack
Chemokines
Small peptide hormone molecules are released to help recruit and attract WBCs to the site of infection
Released due to local inflammation responses caused by DAMPs and PAMPS → cause white cells to leave the circulation and enter areas of inflammation/ damage
E.g. CXCL8 or IL8
Attraction of WBCs is dependent on the cause of inflammation
CXCL8 and IL8
Chemokines that attract neutrophils to inflammed tissues/sites where the pathogen is concerned
Can also cause a similar activation of increasing neutrophil, adhesion, granulation and responsiveness
What happens when the cause of an infection is eliminated in relation to cytokine and chemokine production?
The production of cytokines in the marrow and chemokines in tissues decreases, as the presence of pathogens (PAMPs and DAMPS) that stimulate their production is no longer present.
consequence of self-harm by the innate immune responses
Effects of reduced cytokines and chemokines after pathogen removal
Reduced white cell production: Proliferative compartment dies.
Reduced entry into infected areas: No chemokines present; if cells enter, they are not activated.
Reduced activation: Prevents tissue damage.
Why May Complete Limitation of Self Harm Not Be Possible
White cells use enzymes and other destructive molecules to destroy the organism
The combination of enzymes of different types allows cells to kill all pathogens irrespective of type.
Toxic enzymes are non-selective when in contact with the body
How May Damage By Enzymes be Limited
By targeting them to components in the bacteria e.g. iron-binding protein, or to something that can assist the entry of WBCs e.g. elastase
Aims to limit the WBC response and limit self harm
How May The WBCs Response Be Limited
Killing by WBCs can destroy normal cells, and so there must be a balance between mechanisms to avoid damaging tissues → different cells have different methods to do this
How May The Neutrophil Response Be Limited
WBCs kill the bacteria present within themselves –
No bacteria is released
When the cells undergo apoptosis, they remove granules and enzymes to prevent any damage – localisation in the area of function
Mechanism of Killing Bacteria By Monocytes
Due to their small size, bacteria can be engulfed by these WBCs and digested
Engulf 50-100 bacteria to become full of granules
Granules then fuse with a vacuole containing the bacteria and release them locally – bacteria killed in the vacuoles, which are then apoptosis and disappear
bacteria are destroyed within neutrophils allowing the killing to occur without damage to tissues.
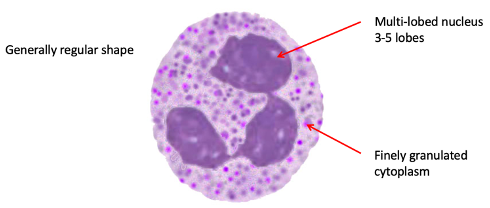
Neutrophil: Resting State
Multilobed nuclei (3-5 lobes)
Finely granulated cytoplasm – ‘killing’ enzymes stored within
Round regular shape
Highly motile cell, which can enter tissues at sites of inflammation
Survives 12-24 hours in blood
Limited lifespan – don’t want half-dead neutrophils; may die by necrosis and release granules - further damage
Further 24-48 hours in tissues
Most neutrophils simply live out their lives and then die without needing to perform infection.
The key therefore is to do nothing unless stimulated this avoids damaging the body.
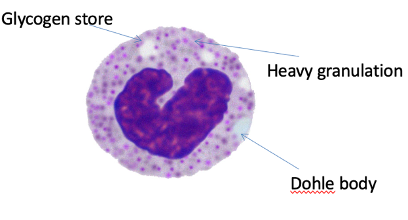
Neutrophil: Activated State
WBCs are released from marrow early – do not have a lobular nucleus
Dohle bodies present – synthesis of proteins to increase the n.o granules present that will be highly active
Highly activated cell – senses surrounding environment – sensitisation – regular outline of cell ready to fight infection
Cytokines and chemokines that are released during inflammation.
These enhance neutrophil function → Enhanced adhesion and movement
Neutrophil Killing Mechanism:
Adhesion to bacteria and vacuole formation
Vacuole and granular fusion to phagosome
Extracellular Traps
Neutrophil Killing Mechanism of Bacteria: Adhesion
Cells bind to the bacteria using adhesion receptors that recognise PAMPS and engulf them into a vacuole
Vacuole then fuses with the phagosome to destroy the bacteria
Neutrophil Killing Mechanism of Bacteria: Role of Granules
They fuse with phagosomes to destroy bacteria through:
Microbiocidal proteins: Myeloperoxidase and lysozyme bind to and destroy proteins (not lipids).
Acid hydrolases: Function only in acidic environments; enzymes become inactive if they escape the vacuole → don’t destroy tissue
Iron binding (Lactoferrin): Binds free iron, preventing bacterial replication, movement, and energy production.
Neutrophil Killing Mechanism of Bacteria: Extracellular Traps
DNA net – final measure if neutrophils haven’t cured infection
As it apoptoses, cells break down their membrane and release its DNA
DNA tangles up and bacteria become tangled and trapped – netosis
How Do Neutrophils Prevent Tissue Damage When KIlling Bacteria
Phagocytoses cells - destroyed internally
Enzyme contents are relatively safe if released: as they depend on low pH or oxidising power which are only found within the cell
Following cell killing neutrophils die by apoptosis avoiding tissue damage
Structure of Monocyte
Kidney shaped nucleus, grey cytoplasms, with fine granules
Large cells
Phagocytic cells with a range of functions
Main function is within tissues - may be regarded as continuing the effects of neutrophils at a later stage – deal within things not dealt with by neutrophils
Generally, irregular shape that is dynamically active contacting other cells or migrating
Spend 17 hours in blood before entering tissues where they become “tissue macrophages”
Can persist for a long time and form a ring/ sphere around any pathogen and wall it off
Function of Monocytes
Phagocytose bacteria
Can “wall in” pathogens (forming a granuloma)
Removes dead cells and promote wound healing
‘Act before the stage of scaring’
Monocyte/ Macrophage Response to Tuberculosis
Celss ‘wall in’ tissue infections
Bacteria can escape death by neutrophils
These cells wall it off to prevent it from moving - seen in lung CT scans - walled off scars seen
Not a permanent solution
Why Isnt the Walling off of TB by monocytes not a permanent solution
As in immunosuppressed individuals, TB can be reactivated
the protective ring of monocytes can break down in response to an insult
Eosinophil and Basophil Mechanism For Killing Larger Organisms
Cells contain numerous granules that fill the cytoplasm and stain strongly with particular acid dyes – appearing red (eosinophil) or blue (basophil)
GRANULES ARE RELEASED IN TISSUES TO DESTROY PARASITES
Must be regulated between killing organisms and preventing excess damage
Granular Contents of Eosinophils
Histamine
Active proteins
Nucleases: breaks down DNA and RNA,
specific, rarely damaging the host
Lipases - break down fat
Not too problematic as they damage fat tissue - worms are fatty
Major basic protein
Contents may potentially cause tissue toxicity as these proteins may damage tissue
Major Basic Protein
Found in the granular contents of eosinophils
A sem-specific substance that attacks an organism substrate and will cause damage to the host when released
Granular Contents of Basophils
They contribute to inflammation
Histamine – dilate blood vessels
Serotonin – dilates blood vessels
Heparin – prevents clotting to allow the entry of immune cells
Keeps work accessible
Enzymes (elastase) break down tissue matrix – vessels more ‘floppy’
IL4 – stimulates immune reactions especially IgE – allows innate immune system to communicate with T-cells
Histamine
Found in the granular contents of eosinophils and basophils
Dialtaes blood vessels, allowing more blood cells to arrive and cause swelling to trap invading organisms
Vessels first constrict tightly, followed by dilation to release fluids into tissue which results in localised swelling → causes tissue to become tight and immobilises the organism
Tissue fluid pressure traps the organism
Inter-Relationships Between The Innate System and The Organism
The response to danger signals produced by organisms allow responses to be proportionate to the level of threat (amount of danger signal) and time (duration of danger signal)
Inter-Relationships Between The Innate System and The Body
Locally active factors such as cytokines and chemokines can be secreted by cells in a range of tissues - this links the immune system to overall health but also allows responses to be localised to sites of threat.
Inter-Relationships Between The Innate System and The Adaptive System
Although arising later in evolutionary terms, the adaptive immune system produces factors that influence the behaviour of the innate system providing extra control
Chediak-Hihashi Syndrome
Low and abnormal granules and nucleus shape of neutrophils
Seen in children – unable to fight infection
Require a bone marrow transplant
includes albinism, recurrent infections, a mild bleeding disorder and peripheral neuropathy.
There are giant granules in the neutrophils, eosinophils, monocytes and lymphocytes, accompanied by neutropenia, thrombocytopenia and marked hepatosplenomegaly.
due to mutations in the CHS1 (LYST) gene, which encodes a lysosomal trafficking regulator.
Immune Neutropenia
An inherited disorder that causes chronic infection due to a low number or defect in the function of neutrophils
Due to an immune attack on these cells
Antibodies directed against Fc gamma receptor IIIb (FcγRIIIb), aka CD16b, found on the surface of neutrophils.
antibodies interfere with phagocytosis, making it difficult for neutrophils to destroy pathogens and leading to an increased infection risk
Rhematiod Artiritis
An inappropriate maintenance of joint inflammation due to high numbers of neutrophils - contributes to damage in inflammatory disease
Drift of fingers due to destruction
Hypereosinophilic Syndrome
A condition characterised by an excess of eosinophils (WBCs), which become over-activated.
This can involve low-level cancer of the eosinophils, leading to excessive histamine release
Eosinophil count elevated above 1.5 × 109/L for over 6 months and associated with tissue damage
heart valves, central nervous system (CNS), skin and lungs may be affected
Mepolizumab a humanised antibody to IL‐5, is a promising new treatment
Consequences of a High Eosinophil Number and Excess Granule Release
It can be harmful, resultin in heart valve damage - ;
Valves deteriorate due to the release of toxic granules in the wrong place
Asthma and Allergy
Caused by an inappropriate number or incorrect circumstance of basophil granule release
Harmful
Hypersensitivity of Basophil in Lung Tissue
Once granule content is released, it causes the swelling of vessels and the compression of small airways - not enough air in the lung
Histamine can cause the airways to become inflamed and narrow, causing wheezing
How May Allergic Responses Be Prevented
By using drugs to stabilise basophils
Suppression of the immune system via steroids
Stabilisation of mast cells e.g. Sodium cromoglicate
Cytokine Storm
The immune system response becomes uncontrolled with cytokine and chemokine production causing excessive activation of inflammatory cells, causing the destruction of normal tissues that further increases cytokine release
This provokes increasing uncontrolled inflammation.
Cytokine Storm in COVID-19 Patients
Seen in severely affected individuals, where the normal response to a viral infection by B and T lymphocytes became uncontrolled, with increasing cytokine release that provokes inflammation due to inappropriate activation of the innate immune system.
Dexamethasone
An example of a steroid that can be used at an early stage to suppress inflammation and prevent the progression of cytokine storms
Had major impacts on COVID-19 disease progression