Biochem - 10.1 (book + lect notes)
1/294
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
295 Terms
Q: Where are the enzymes of the citrate cycle and electron transport system located in eukaryotic cells?
A: All enzymes in the citrate cycle and electron transport system reside in the mitochondrial inner membrane and matrix.
Q: What occurs in the mitochondrial matrix related to pyruvate?
A: Pyruvate is converted to acetyl-CoA by the enzyme pyruvate dehydrogenase.
Q: Aside from glycolysis, how else can pyruvate and acetyl-CoA be derived?
A: They can be derived from amino acid catabolism.
Q: How else is acetyl-CoA produced besides glycolysis or amino acid catabolism?
A: Acetyl-CoA is produced by fatty acid oxidation and serves as a major energy source in most organisms.
Q: What is the primary function of the citrate cycle?
A: To oxidize acetyl-CoA.
Q: How many pairs of electrons are transferred during each turn of the citrate cycle?
A: Four pairs of electrons (8 e⁻) are transferred from citrate cycle intermediates to NAD⁺ and FAD, generating 3 NADH and 1 FADH₂.
Q: How can the citrate cycle be described metaphorically?
A: It can be thought of as a “metabolic engine” where the fuel is acetyl-CoA, the exhaust is CO₂, and the work performed is electron transfer through redox reactions.
Q: What type of phosphorylation occurs in the citrate cycle?
A: A substrate-level phosphorylation reaction generates 1 GTP per turn, which nucleoside diphosphate kinase uses to phosphorylate ADP to ATP.
Q: What is the function of oxidative phosphorylation in mitochondria?
A: To generate about 5 ATP for every 2 NADH oxidized.
Q: What is the ATP yield per NADH molecule?
A: Approximately 2.5 ATP per NADH.
Q: How much ATP results from oxidizing 2 FADH₂ molecules?
A: Around 3 ATP total (~1.5 ATP per FADH₂).
Q: How many ATP are produced per turn of the citrate cycle?
A: About 10 ATP for each acetyl-CoA molecule oxidized (including substrate-level phosphorylation).
Q: What happens during oxidation of NADH and FADH₂ by the electron transport system?
A: Electrons are transferred to O₂, forming H₂O.
Q: Why is regeneration of NAD⁺ and FAD important?
A: It maintains flux through the citrate cycle since several enzymes require NAD⁺ or FAD as coenzymes.
Q: Why does the metabolic engine of the citrate cycle require oxygen?
A: Because continuous redox reactions depend on a constant O₂ supply, similar to a combustion engine.
Q: How is the citrate cycle similar to glycolysis under anaerobic conditions?
A: Both rely on regeneration of NAD⁺—glycolysis regenerates NAD⁺ via the glyceraldehyde-3-P dehydrogenase reaction under anaerobic conditions.
Q: Who first described the citrate cycle and when?
A: Hans Krebs in 1937.
Q: What early observations led to the discovery of the citrate cycle?
A: Experiments showed that minced muscle tissues oxidized organic acids (citrate, fumarate, malate, succinate) in the presence of oxygen to produce CO₂.
Q: What did addition of malate or oxaloacetate to muscle cells cause?
A: It stimulated oxygen reduction to H₂O at much higher rates than needed for the added substrate, indicating a cyclic pathway.
Q: What was discovered about urea synthesis by Krebs and Henseleit?
A: Urea is synthesized from amino acids and ammonia in a cyclic pathway involving both cytosolic and mitochondrial enzymes.
Q: What idea linked the urea cycle to the citrate cycle discovery?
A: The hypothesis that formation of oxaloacetate from malate and formation of citrate from pyruvate are connected cyclic reactions.
Q: What compound helped Krebs confirm the cyclic nature of the citrate cycle?
A: Malonate, which inhibits succinate dehydrogenase and causes accumulation of cycle intermediates.
Q: Who shared the 1953 Nobel Prize with Krebs for discovering the role of acetyl-CoA?
A: Fritz Lipmann.
Q: What other names are used for the citrate cycle?
A: The Krebs cycle and the tricarboxylic acid (TCA) cycle.
Q: Why is it called the tricarboxylic acid (TCA) cycle?
A: Because citrate, the first product, is a tricarboxylate (conjugate base of citric acid).
Q: What are the pKa values of the three carboxyl groups in citric acid?
A: 3.1, 4.7, and 6.4.
Q: What two features distinguish the citrate cycle from linear pathways like glycolysis?
A:
Oxaloacetate is both the substrate for the first reaction (catalyzed by citrate synthase) and the product of the last (catalyzed by malate dehydrogenase).
The cycle regenerates oxaloacetate each turn, maintaining intermediate concentrations and boosting capacity for acetyl-CoA oxidation and CO₂ production.
Q: What are the two enzymes that control the flow of acetyl-CoA and oxaloacetate into the citrate cycle?
A: Pyruvate dehydrogenase and pyruvate carboxylase.
Q: Why are pyruvate dehydrogenase and pyruvate carboxylase discussed with citrate-cycle enzymes?
A: Because they play auxiliary roles controlling substrate entry into the cycle.
Q: When is pyruvate dehydrogenase inhibited?
A: When ATP and NADH levels are high, signaling high cellular energy.
Q: What happens to excess acetyl-CoA when energy levels are high?
A: It is diverted to fatty acid synthesis.
Q: How does the relationship between carbohydrate and fatty acid metabolism explain low-carb diets?
A: Limiting carbohydrates triggers fat metabolism to produce acetyl-CoA for the citrate cycle.
Q: What does the citrate cycle accomplish for the cell?
A: Transfers 8 electrons from acetyl-CoA to NAD⁺ and FAD, forming 3 NADH and 1 FADH₂, which produce ATP via oxidative phosphorylation; generates 2 CO₂ and 1 GTP; and provides intermediates for gluconeogenesis, amino acid, and porphyrin biosynthesis.
Q: What is the overall net reaction of the citrate cycle?
Acetyl-CoA + 3 NAD⁺ + FAD + GDP + Pᵢ + 2 H₂O → CoA + 2 CO₂ + 3 NADH + 3 H⁺ + FADH₂ + GTP
Q: What enzyme regulates the flux of acetyl-CoA through the cycle?
A: Pyruvate dehydrogenase.
Q: How is pyruvate dehydrogenase regulated?
A: Activated by NAD⁺, CoA, and Ca²⁺; inhibited by acetyl-CoA, ATP, and NADH.
Q: What enzyme catalyzes the first reaction of the citrate cycle?
A: Citrate synthase.
Q: What inhibits citrate synthase?
A: Citrate, succinyl-CoA, NADH, and ATP.
Q: What does isocitrate dehydrogenase do?
A: Catalyzes oxidative decarboxylation of isocitrate, transferring electrons to NAD⁺ to form NADH and releasing CO₂.
Q: How is isocitrate dehydrogenase regulated?
A: Activated by ADP and Ca²⁺; inhibited by NADH and ATP.
Q: What is α-ketoglutarate dehydrogenase’s role?
A: Performs oxidative decarboxylation producing CO₂, NADH, and succinyl-CoA.
Q: How is α-ketoglutarate dehydrogenase regulated?
A: Activated by Ca²⁺ and AMP; inhibited by NADH, succinyl-CoA, and ATP.
(Example) Q: What is an example of the citrate cycle in everyday biochemistry?
A: Fluoroacetate, a toxin found in some plants and used in Compound 1080, is converted in cells to fluorocitrate, which inhibits mitochondrial aconitase and causes cell death.
Q: What is a redox reaction in metabolism?
A: A form of energy conversion involving electron transfer from organic substrates to carrier molecules.
Q: What determines the energy available from redox reactions?
A: Differences in electron affinity of the reacting compounds.
Q: Why can’t electrons exist freely in solution?
A: They must transfer between compounds in redox reactions.
Q: What two half-reactions make up each redox reaction?
A: (1) Oxidation (loss of electrons) and (2) Reduction (gain of electrons).
Q: What are oxidants and reductants?
:
Oxidants (oxidizing agents): Accept electrons and are reduced.
Reductants (reducing agents): Donate electrons and are oxidized.
Q: What mnemonic helps remember oxidation and reduction?
A: “OIL RIG” — Oxidation Is Loss (of electrons); Reduction Is Gain (of electrons).
Alternate: “LEO the lion says GER.”
Q: What organelles contain the citrate cycle and electron transport enzymes in eukaryotic cells?
A: The mitochondria — specifically the inner membrane and matrix.
Q: Why don’t prokaryotic cells perform the citrate cycle the same way as eukaryotes?
A: They lack metabolically active organelles like mitochondria.
Q: What is the overall “engine analogy” for the citrate cycle as shown in Figure 10.3?
A: The citrate cycle acts as a metabolic engine where:
Fuel: Acetyl-CoA
Exhaust: CO₂
Work: Production of GTP and reduction of NAD⁺/FAD to NADH/FADH₂
Energy output: Used to power ATP synthesis via oxidative phosphorylation.
Q: What happens to the reducing equivalents (NADH, FADH₂) generated by the citrate cycle?
A: They enter the electron transport system, transferring electrons that drive ATP synthesis through oxidative phosphorylation.
Q: How does ATP synthase produce ATP?
A: It uses the proton gradient created by electron transport to synthesize ATP from ADP and Pᵢ.
Q: What enzyme converts the GTP made in the citrate cycle into ATP?
A: Nucleoside diphosphate kinase.
Q: Why is the citrate cycle considered “cyclic” rather than linear?
A: Because oxaloacetate, the first substrate, is regenerated in the final step, allowing continuous operation without net consumption of intermediates.
Q: What was the significance of Krebs’ discovery for metabolic theory?
A: It revealed that cellular energy production follows cyclic pathways instead of linear ones, allowing continual regeneration of key intermediates.
Q: What do redox reactions in biochemistry typically involve?
A: They rarely involve molecular oxygen (O₂) directly but are characterized by the loss and gain of electrons from carbon.
Q: How is biochemical redox reaction terminology similar to inorganic chemistry?
A: Each half-reaction consists of a conjugate redox pair—a molecule with and without an electron (e⁻).
Q: What is an example of a conjugate redox pair?
A: Fe²⁺/Fe³⁺ is a conjugate redox pair where Fe²⁺ is the reductant that loses an electron to become Fe³⁺, the oxidant.
Equation: Fe²⁺ ⇌ Fe³⁺ + e⁻
Q: What is another example of a conjugate redox pair?
A: Cu⁺/Cu²⁺, where Cu⁺ is the reductant that loses an e⁻ to form Cu²⁺, the oxidant.
Equation: Cu⁺ ⇌ Cu²⁺ + e⁻
Q: What are the conjugate redox pairs in Fe²⁺/Fe³⁺ and Cu⁺/Cu²⁺ systems?
A: Fe²⁺/Fe³⁺ and Cu⁺/Cu²⁺.
Q: How can these redox pairs be combined into a coupled redox reaction?
A: By reversing the Fe³⁺ reduction reaction so that the e⁻ serves as a shared intermediate between Cu⁺ oxidation and Fe³⁺ reduction.
Equations:
Fe³⁺ + e⁻ ⇌ Fe²⁺ (reduction)
Cu⁺ ⇌ Cu²⁺ + e⁻ (oxidation)
Fe³⁺ + Cu⁺ ⇌ Fe²⁺ + Cu²⁺ (coupled redox reaction)
Q: What metal ions are involved in redox reactions of the electron transport system?
A: Fe²⁺/Fe³⁺ and Cu⁺/Cu²⁺.
Q: How do redox reactions in the citrate cycle differ from Fe³⁺/Cu⁺ reactions?
A: Citrate cycle redox reactions transfer pairs of electrons (2 e⁻) to electron carriers NAD⁺ and FAD, not single electrons.
Q: How is NAD⁺ reduced to NADH + H⁺?
A: The reductant donates two hydrogen atoms—one as a hydride ion (H⁻, containing 2 e⁻ and 1 H⁺) and one as a proton (H⁺).
Equation: NAD⁺ + :H⁻ + H⁺ ⇌ NADH + H⁺
Q: How can this NAD⁺ reduction reaction be alternatively written?
A: NAD⁺ + 2 e⁻ + 2 H⁺ ⇌ NADH + H⁺
Q: How is FAD reduced to FADH₂?
A: By the sequential addition of one hydrogen (1 e⁻ + 1 H⁺) at a time.
Equations:
FAD + 1 e⁻ + 1 H⁺ ⇌ FADH•
FADH• + 1 e⁻ + 1 H⁺ ⇌ FADH₂
Q: How can oxidation involve oxygen directly?
A: Oxygen pulls electrons from carbon toward itself, oxidizing the carbon due to oxygen’s high electronegativity.
Q: What are enzymes that catalyze redox reactions called?
A: Oxidoreductases.
Q: What are oxidation reactions involving loss of hydrogen often catalyzed by?
A: Dehydrogenases.
Q: What are the two primary energy-conversion reactions in metabolism?
A: (1) Phosphoryl transfers involving ATP and (2) redox reactions using NAD⁺/NADH and FAD/FADH₂.
Q: How can energy conversion reactions be compared?
A: By comparing their changes in standard free energy (ΔG°′).
Q: What does the biochemical standard free energy change (ΔG°′) measure?
A: The spontaneity of a reaction—its tendency to proceed under standard conditions.
Q: What are the units of ΔG°′ and what do they represent?
A: Kilojoules per mole (kJ/mol), representing the energy change for 1 M concentrations of reactants and products.
Q: What is the biochemical standard reduction potential (E°′)?
A: A measure (in volts) of a conjugate redox pair’s electron affinity, analogous to standard Gibbs free energy (ΔG°′).
Q: How are E°′ values interpreted?
A:
Oxidants with higher electron affinity than H⁺ → positive E°′ (> 0)
Oxidants with lower electron affinity than H⁺ → negative E°′ (< 0)
Q: How is a standard hydrogen electrode used as a reference?
A: It has an assigned E°′ value of 0.00 V. Comparing this to the Fe²⁺/Fe³⁺ cell gives a measured E°′ of +0.77 V, indicating Fe³⁺ has higher electron affinity than H⁺.
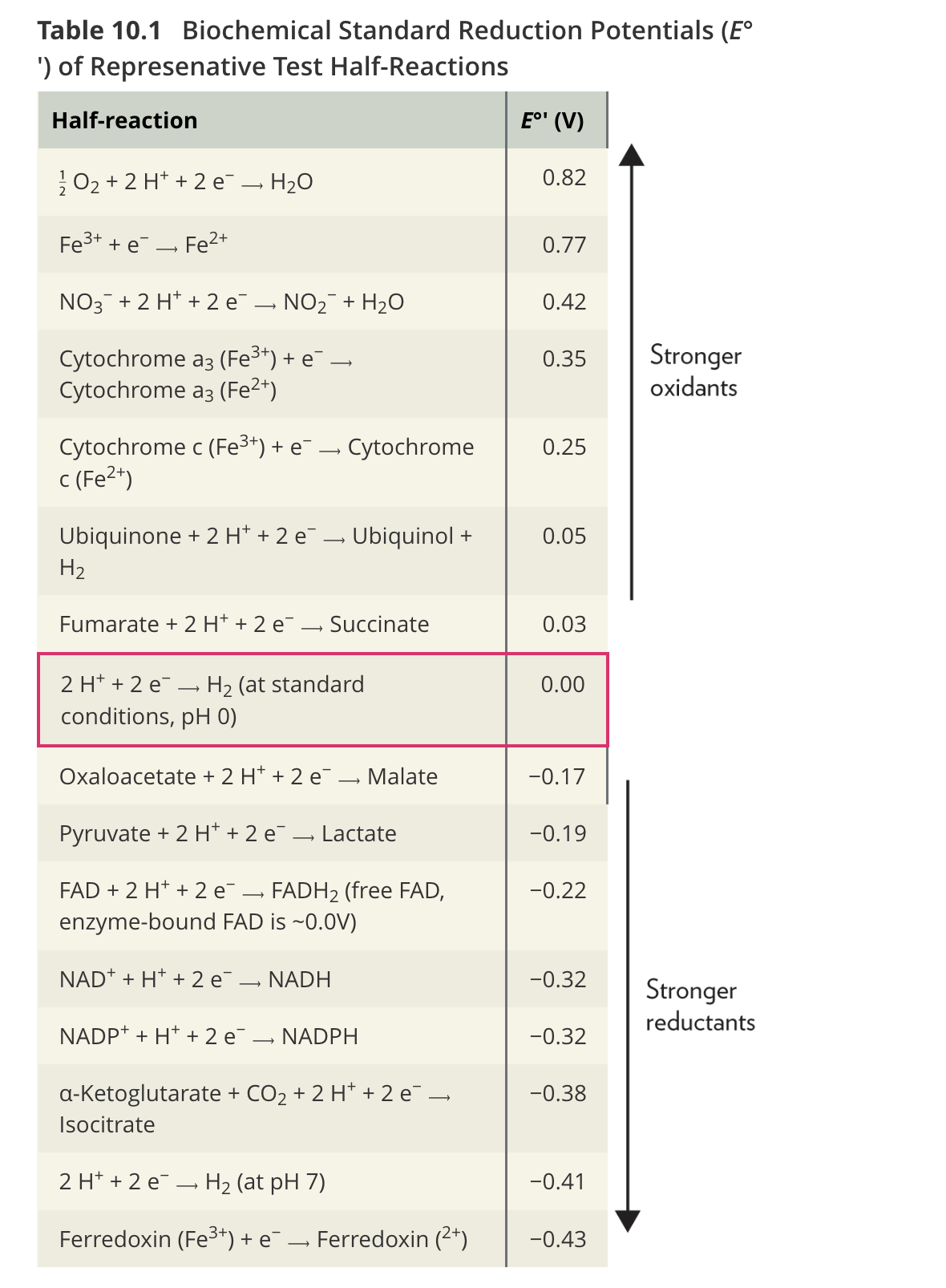
Q: What does Table 10.1 show about O₂ and ferredoxin?
A: O₂ is the most potent oxidant (E°′ = +0.82 V), while ferredoxin (containing Fe³⁺) is the weakest oxidant but strongest reductant.
Q: How are standard reduction potentials expressed?
A: As half-reactions written in the direction of reduction.
Q: What is the standard reduction potential of hydrogen at pH 7?
A: E°′ = –0.41 V, meaning electrons tend to move from pH 7 medium to the pH 0 reference electrode.
Q: What determines the amount of energy available from a coupled redox reaction?
A: The difference between the reduction potentials of the oxidant and the reductant (ΔE°′).
Equation:
ΔE°′ = (E°′_oxidant) – (E°′_reductant)
Q: How is ΔE°′ related to standard free energy change (ΔG°′)?
Equation: ΔG°′ = –nFΔE°′
where n = number of electrons transferred, and F = Faraday constant (96.485 kJ/V·mol).
Q: When is a coupled redox reaction favorable?
: When ΔE°′ > 0, then ΔG°′ < 0 — meaning the reaction is spontaneous.
Q: How are ΔG°′ and ΔE°′ used together in biochemical calculations?
A: Using E°′ values from a table, you can calculate ΔG°′ for reactions like the isocitrate dehydrogenase reaction.
Equation Example:
Isocitrate + NAD⁺ ⇌ α-Ketoglutarate + CO₂ + NADH + H⁺
Q: In spontaneous redox reactions, how do electrons flow?
A: From the reductant with the lower (more negative) E°′ to the oxidant with the higher (less negative) E°′.
Q: What are the half-reactions for isocitrate dehydrogenase?
Equations:
α-Ketoglutarate + CO₂ + 2 e⁻ + 2 H⁺ → Isocitrate (E°′ = –0.38 V)
NAD⁺ + 2 e⁻ + 2 H⁺ → NADH + H⁺ (E°′ = –0.32 V)
Q: How do you calculate ΔE°′ for this reaction?
Equations:
ΔE°′ = (E°′_acceptor) – (E°′_donor)
ΔE°′ = (E°′_NAD⁺) – (E°′_isocitrate)
ΔE°′ = (–0.32 V) – (–0.38 V) = +0.06 V
Q: How do you calculate ΔG°′ from ΔE°′?
Equations:
ΔG°′ = –nFΔE°′
ΔG°′ = –(2)(96.485 kJ/V·mol)(+0.06 V)
ΔG°′ = –11.6 kJ/mol
Q: What does this calculation show about isocitrate → α-ketoglutarate conversion?
A: It is favorable (ΔG°′ < 0) under standard biochemical conditions.
Q: Why do actual mitochondrial conditions differ from standard biochemical conditions?
A: Because substrate and product concentrations inside mitochondria vary, affecting reduction potentials.
Q: Which equation accounts for these nonstandard conditions?
A: The Nernst equation (Equation 10.3).
Equation:
E = E°′ + (RT / nF) ln([e⁻ acceptor] / [e⁻ donor])
Q: What do R, T, n, and F represent in the Nernst equation?
A:
R: Gas constant (8.314 × 10⁻³ kJ/mol·K)
T: Temperature (K)
n: Number of electrons transferred
F: Faraday constant (96.485 kJ/V·mol)
Q: How is the Nernst equation used for the NAD⁺/NADH redox pair?
A: By applying the experimentally determined 20:1 ratio of NAD⁺ to NADH in mitochondria.
Calculation:
E = –0.32 V + (0.013)(3) = –0.28 V
Q: What does the actual reduction potential of –0.28 V indicate?
A: It is more positive than the standard –0.32 V, meaning NAD⁺ reduction is even more favorable inside mitochondria due to a high NAD⁺/NADH ratio.
Q: What do redox reactions accomplish in metabolism?
A: They enable energy transfer by coupling oxidation of fuels to reduction of electron carriers like NAD⁺ and FAD, which later drive ATP synthesis.
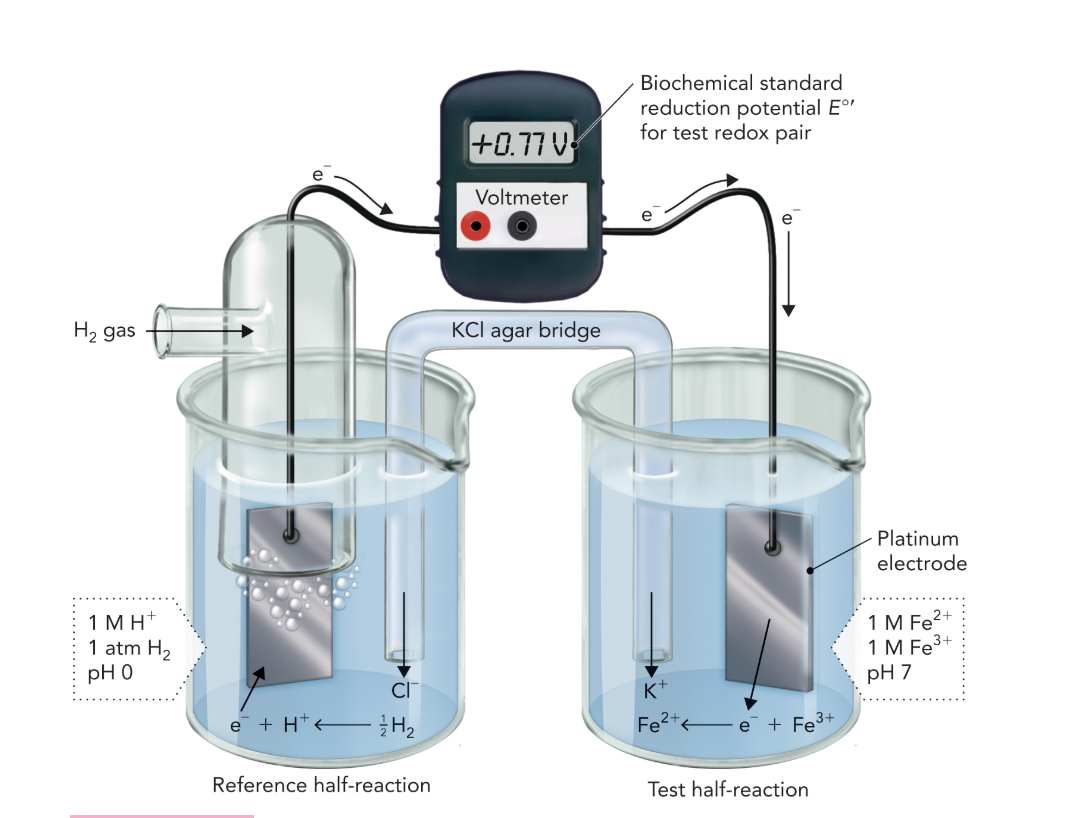
Q: What does Figure 10.5 demonstrate about electron flow?
A: Electrons flow spontaneously from the redox pair with a more negative E°′ (donor) to one with a more positive E°′ (acceptor), indicating direction of favorable energy transfer.
Q: What does a ΔE°′ of +0.06 V signify in energetic terms?
A: A small positive potential difference corresponds to a modest but favorable energy release (~11.6 kJ/mol) sufficient to drive part of the citrate-cycle turnover.
Q: Why are differences in reduction potentials biologically important?
A: They create the electron-transfer “pressure” that fuels the electron-transport chain, proton pumping, and ultimately ATP formation.
Q: Who introduced the Nernst equation and why is it important?
A: Walther Nernst (1881); it allows calculation of real-cell potentials under non-standard biochemical conditions, vital for understanding mitochondrial energetics.