Topic 14- GLucose Metabolism
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
Glucose At Heart of Metabolism pt1
Metabolism of glucose is at heart of metabolism
A. Major metabolic fuel for all body cells
B. Average body consumption ~ 160 – 200 g/day
II. Brain is major consumer of glucose (~ 120 g/day)
A. Glucose is its major energy source
1. Cannot synthesize glucose & can only
store a limited amount
Glucose At Heart of Metabolism pt2
I. RBCs absolutely require glucose
A. Do not possess mitochondria & cannot do aerobic metabolism
1. Depend exclusively on anaerobic metabolism for energy needs
II. Approximate body glucose reserves (in 70 kg man after overnight fast)
A. Extracellular glucose ~ 4 – 5 g
B. Liver glycogen ~ 80 g
C. Muscle glycogen ~ 150 g
Regulation of Blood Glucose Levels
I. Blood glucose levels must be tightly maintained
A. Due to high body demand vs. moderate body reserves
B. [Glucose]Blood = 70 – 100 mg/dL
II. 2 key organs regulate [glucose]Blood
A. Pancreas: produces hormones regulating glucose metabolism
B. Liver: major site of glucose metabolism
Hyperglycemia pt1
I. High [glucose]Blood
A. Normally occurs after the consumption of a carbohydrate-rich meal
II. beta cells of pancreas produce & secrete the hormone insulin
A. Insulin stimulates glucose uptake from blood into muscle, adipose tissue, heart, & liver
Hyperglycemia pt2
Insulin-stimulated ↑ [glucose]Intracellular is dealt with in 2 ways
A. Glycolysis (in all cells): breakdown & utilization of glucose
B. Glycogenesis (in liver & skeletal muscle): storage of glucose as glycogen (glucose uptake + storage)
Hypoglycemia pt1
I. Low [glucose]Blood
A. Normally occurs ~ 4 hours after end of last meal or during prolonged, intense physical activity that increases metabolic demand
II. alpha cells of pancreas produce & secrete the hormone glucagon (liberation and release of glucose)
A. Glucagon stimulates liver to produce more glucose for release into blood
Hypoglycemia pt2
I. Increased glucose demand is met in 2 ways
A. Glycogenolysis (in liver & skeletal muscle): liberation of glucose from glycogen
1. Liver does this to release glucose into blood
2. Muscle does this to release glucose for its own use (skeletal m.)
B. Gluconeogenesis (in liver & kidneys): production of glucose from noncarbohydrate precursors
1. Produced glucose is released into blood
Glucose in Fight-or-Flight Response
During this response, body needs ready access to adequate amounts of metabolic fuel
A. Adrenal glands produce & secrete the hormone
epinephrine1. Targets liver & skeletal muscle
i. In liver, stimulates glycogenolysis & gluconeogenesis, followed by glucose release into blood
ii. In skeletal muscle, stimulates glycogenolysis & glycolysis to facilitate muscle action
Glycogenesis
I. Synthesis of glycogen
A. Occurs through sequential, 1-at-a-time addition of glucose monomers to nonreducing ends of glycogen
𝐆𝐥𝐮𝐜𝐨𝐬𝐞 + 𝐆𝐥𝐲𝐜𝐨𝐠𝐞𝐧(𝐧 𝐠𝐥𝐮𝐜𝐨𝐬𝐞 𝐮𝐧𝐢𝐭𝐬) —𝐨𝐧𝐭𝐨 𝐍𝐨𝐧𝐫𝐞𝐝𝐮𝐜𝐢𝐧𝐠 𝐄𝐧𝐝𝐬—> 𝐆𝐥𝐲𝐜𝐨𝐠𝐞𝐧 (n + 𝟏 𝐠𝐥𝐮𝐜𝐨𝐬𝐞 𝐮𝐧𝐢𝐭𝐬)
B. Also involves formation of branches in
glycogen molecule
Glycogenesis: Step 1
I. Catalyzed by hexokinase (muscle) & glucokinase
(liver)A. 1st step of glycolysis: ATP-Mg2+-mediated phosphorylation of glucose to produce G6P
1. Remember G6P is branch point connection of metabolism
i. Instead of continuing through glycolysis a different metabolic branch
will be chosen
Glycogenesis: Step 2
Catalyzed by phosphoglucomutase
A. Isomerizes G6P into glucose-1-
phosphate (G1P)

Glycogenesis: Step 3
Catalyzed by UDP-glucose pyrophosphorylase
A. Combines G1P with UTP to produce uridine diphosphate glucose (UDP-
glucose; UDPG)1. “High-energy” compound that supplies G to drive glycogenesis
B. To drive this reaction, UTP is hydrolyzed to UMP & PPi (1st high-energy
bond cleavage), followed by PPi hydrolysis into 2 Pi by inorganic
pyrophosphatase (2nd high-energy bond cleavage)

Glycogenesis: Step 4
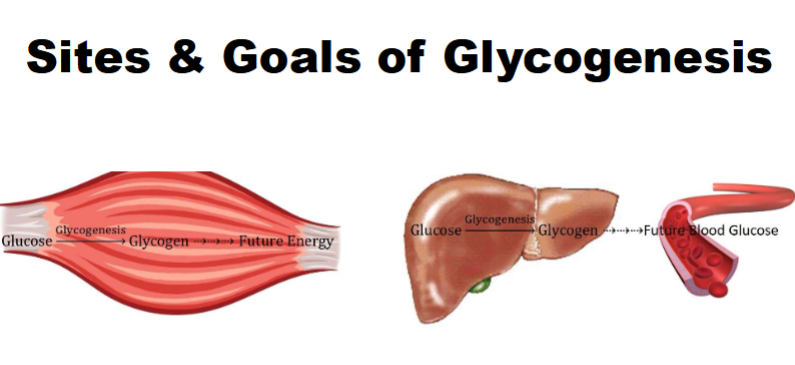
Catalyzed by glycogenin
A. Responsible for synthesis of nascent (i.e., brand new) glycogen molecules
B. Hydrolyzes UDPG to produce UDP & glucose bound directly to enzyme
C. Hydrolyzes next UDPG to produce UDP & 2nd glucose bound to 1st
glucosyl residue via alpha(1→4) glycosidic bondD. Repeats this reaction until a chain of 7 – 9 glucosyl residues is made; it then passes chain off to next enzyme
Glycogenesis: Step 5
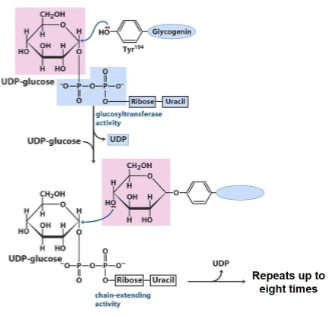
Catalyzed by glycogen synthase (glycogen synthase 1 in muscle; glycogen synthase 2 in liver)
A. Receives glycogen primer from glycogenin
1. Cannot synthesize nascent chains, can only extend pre-existing glycogen molecules
B. Hydrolyzes UDPG to produce UDP & glucose attached to a nonreducing end of glycogen chain via
alpha(1→4) glycosidic bond1. Reaction is repeated until all available UDPG are used up
C. Rate-limiting step of glycogenesis
Glycogenesis: Step 5.5
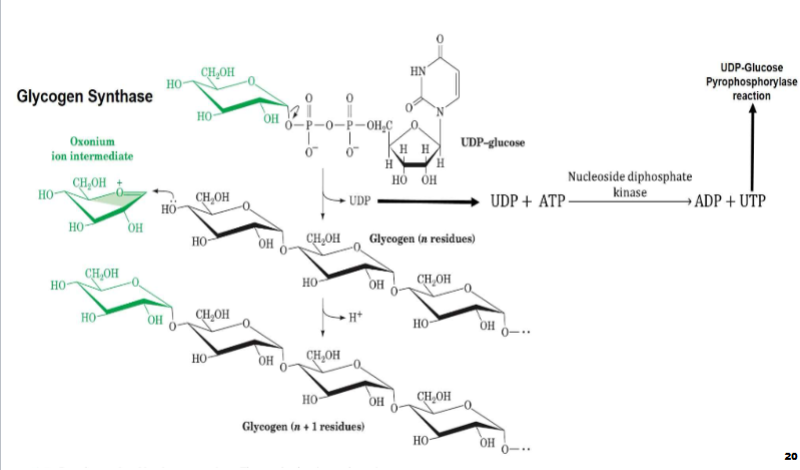
I. Bc UTP hydrolysis drives this process, liberated UDP from glycogenin & glycogen synthase reactions must be reconverted to
UTPA. Nucleoside diphosphate kinase uses ATP to phosphorylate these UDP molecules to produce UTP & ADP
Glycogenesis: Step 6
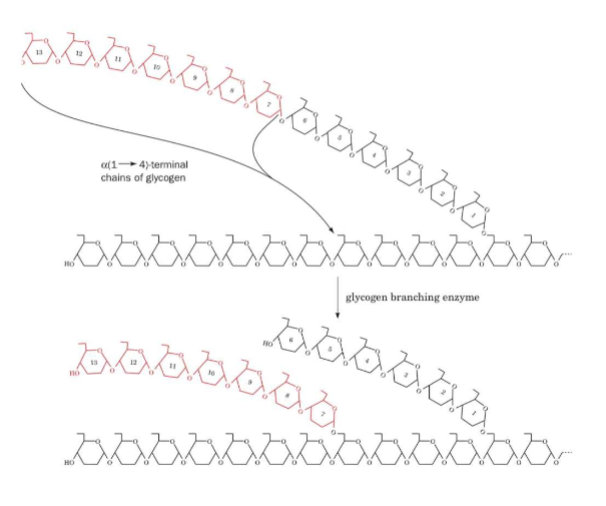
Catalyzed by glycogen branching enzyme
A. Cleaves a terminal heptaglucosyl segment off nonreducing end of a glycogen chain that is at least 11 glucosyl residues long
B. Attaches heptaglucosyl segment to an internal glucosyl residue of a glycogen chain via alpha(1→6) glycosidic bond
1. New branch must be at least 4 glucosyl residues away from other branches
i. Glycogen synthase can extend length of these branches
Glycogenolysis
Breakdown of glycogen
A. Occurs through sequential, 1-at-a-time removal of individual glucose monomers from nonreducing ends of glycogen
𝐆𝐥𝐲𝐜𝐨𝐠𝐞𝐧(𝐧 𝐠𝐥𝐮𝐜𝐨𝐬𝐞 𝐮𝐧𝐢𝐭𝐬) —𝐟𝐫𝐨𝐦 𝐍𝐨𝐧𝐫𝐞𝐝𝐮𝐜𝐢𝐧𝐠 𝐄𝐧𝐝𝐬—> 𝐆𝐥𝐲𝐜𝐨𝐠𝐞𝐧 (n- 𝟏 𝐠𝐥𝐮𝐜𝐨𝐬𝐞 𝐮𝐧𝐢𝐭𝐬) + 𝐆𝐥𝐮𝐜𝐨𝐬𝐞
B. Highly branched structure allows for rapid release of glucose units from end of every branch
C. Also involves the debranching of glycogen molecule
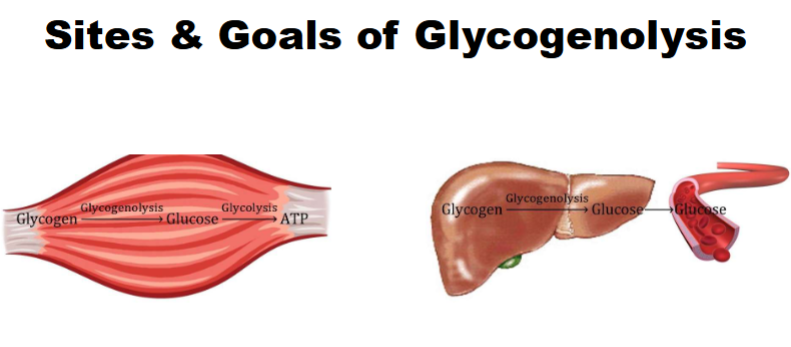
Glycogenolysis: Step 1
Catalyzed by glycogen phosphorylase
A. Utilizes pyridoxal phosphate (PLP) cofactor & Pi to cleave terminal alpha (1→4) glycosidic bond in a nonreducing end of glycogen to liberate a G1P
molecule1. Will only cleave off glucosyl residue if it is more than 4 glucosyl residues away from a branch point
B. Reaction will occur over & over again until glucose needs are met
C. Rate-limiting step of glycogenolysis
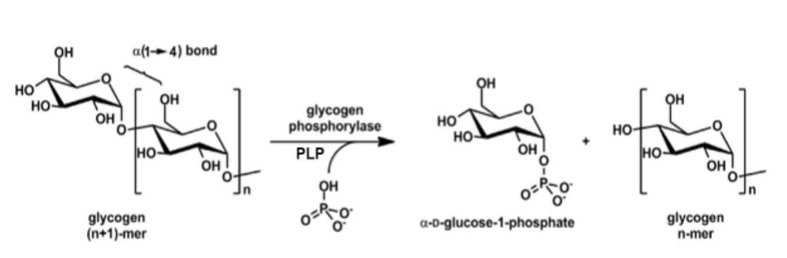
Glycogenolysis: Step 2
Catalyzed by glycogen debranching enzyme
A. Possesses 2 enzymatic functions
1. Works on limit branches (tetraglucosyl unit) by cleaving alpha(1→4) glycosidic bond between branch point glucosyl residue & 2nd glucosyl residue of limit branch
i. Transfers liberated triglucosyl unit to a nonreducing end of glycogen (this allows glycogen phosphorylase to break it down)
2. Liberates branch point glucosyl residue by cleaving alpha(1→6) glycosidic bond, releasing it as glucose
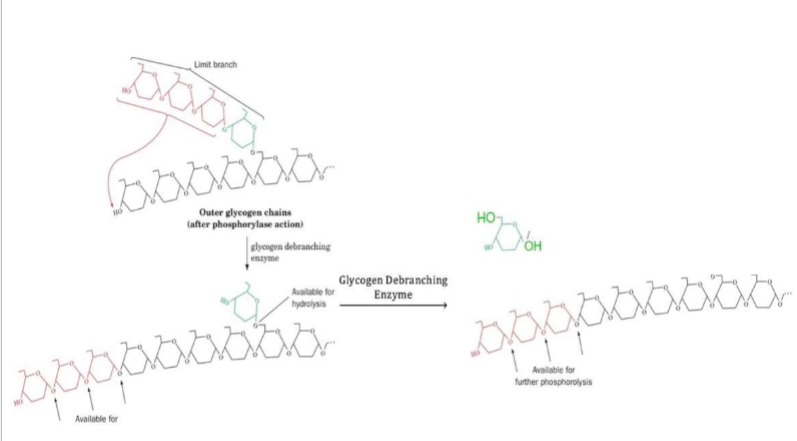
Glycogenolysis: Step 3
Glycogenolysis: Step 3
I. Catalyzed by phosphoglucomutase (same enzyme as in glycogenesis)
A. Performs reverse reaction to isomerize G1P into G6P
1. In muscle, G6P & glucose liberated from glycogen will feed directly into glycolysis for energy production
2. Additional steps are needed in liver
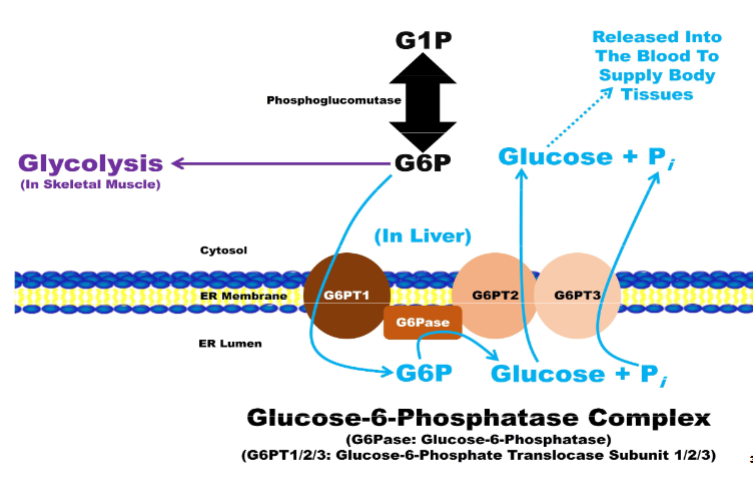
Glycogenolysis: Additional Steps In Liver pt1
G6P imported into hepatocyte ER lumen to beacted upon by glucose-6-phosphatase (G6Pase) enzyme complex
A. Composed of 4 subunits
1. G6P translocase subunit 1 (G6PT1): transports G6P from cytosol into ER lumen
2. G6Pase: catalytic subunit that dephosphorylates G6P to produce glucose &
Pi
Glycogenolysis: Additional Steps In Liver (2)
Composed of 4 subunits (continued...)
A. G6P translocase subunit 2 (G6PT2): transports glucose from ER lumen into
cytosolB. G6P translocase subunit 3 (G6PT3): transports Pi from ER lumen into cytosol
II. Glucose exported into blood through GLUT2
Regulation of Glycogen Metabolism
I. Major points of control are the rate-limiting steps
A. Glycogenesis: glycogen synthase
B. Glycogenolysis: glycogen phosphorylase
II. Control of these processes is primarily driven by action of insulin, glucagon, & epinephrine
Allosteric Regulation of Glycogen Metabolism
I. Liver
A. Glucose: allosteric inhibitor of glycogen phosphorylase a
B. G6P: allosteric activator of glycogen synthase b & PP1
II. Muscle
A. G6P: allosteric activator of glycogen synthase b & PP1; allosteric inhibitor of glycogen phosphorylase b
B. AMP: allosteric activator of glycogen phosphorylase b
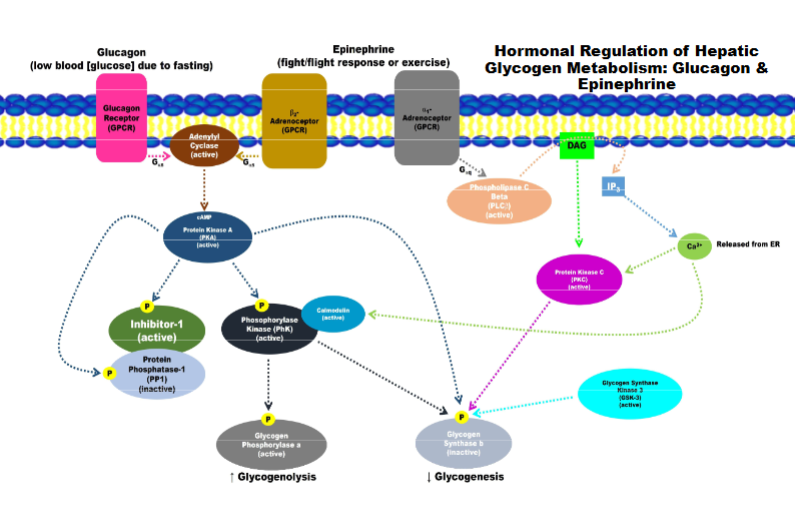
Hormonal Regulation of Hepatic Glycogen Metabolism: Glucagon and Epinephrine
Signalling for break down of glyco and preventing synthesis of glyco
a is active for b is inactive/inhibited
phosphorylation and binding of other protein to turn PP1 off
activating breakdown of glycogen and inhibit its synthesis
Hormonal Regulation of Hepatic & Muscle Glycogen Metabolism: Insulin
epi primary hormone receptor
same mechanism as other one except with muscle mechanism AMPK (its a kinase sensitive to amp levels
amp allosterically active ampk (extra mechanism to make sure glycogen happens)
JOb of enzyme to degrade secong messanger
whole job: active glycogen synthesis and inhibit glycogen breakdown
feedback inhibition stronger in liver than in muscle

Why so much metabolic effort to use glycogen for G
storage when fatty acids are far more abundant in
body & are richer source of G?(Glycogen vs Fat)
A. Muscles cannot mobilize FA (Fatty acid) as rapidly as glycogen
B. FA cannot be metabolized anaerobically, but glucose can
C. FA cannot fuel brain or RBCs
D. FA cannot be converted into glucose, thus they cannot maintain proper [glucose]Blood
Glycogen Storage Diseases (GSD) pt1
I. Rare recessively inherited disorders caused by mutations in enzymes involved in glucose metabolism (glycolysis & gluconeogenesis) &/or glycogen
metabolism (glycogenesis & glycogenolysis)II. Typically leads to abnormal accumulation of glycogen (either too little or too much glycogen)
III. Specific mutations affect specific organs
A. There are hepatic GSD (affecting liver), myopathic GSD (affecting muscle), & generalized GSD (affecting most organs)
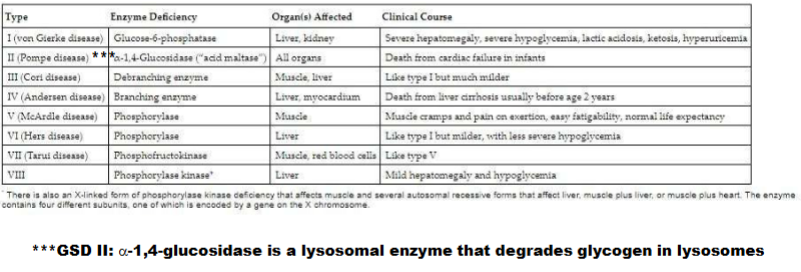
Glycogen Storage Diseases pt 2
Know the names, the enzymes that are effected
Need for Other Sources of Glucose pt1
Under fasting/starvation conditions, continuous vigorous exercise, or consumption of a low carbohydrate diet, glycogen can only supply a
portion of the glucose that body (especially the brain) needs to functionA. This portion decreases as period of fasting/starvation, vigorous exercise, or
consumption of low carb diet increases in duration
Need for Other Sources of Glucose pt2
I. Fasting conditions: glycogen depleted within 20 – 30 hours after last meal
II. Continuous vigorous exercise: glycogen depleted within 2 – 3 hours (e.g., running marathon)
III. Low carbohydrate diet: glycogen stores perpetually low/depleted
IV. In these scenarios, body turns to gluconeogenesis to supply glucose
Gluconeogenesis
I. Conversion of noncarbohydrate precursors into glucose
II. Occurs primarily in liver, but also in kidneys in late stages of fasting/starvation
III. Utilizes reverse reactions of glycolytic enzymes (occurs in cytosol)
A. Exception are 3 rate-limiting enzymes of glycolysis
1. Different enzymes must be used; these are referred to as bypass reactions
Gluconeogenic Building Blocks pt1
Several noncarbohydrate precursors can be used to make glucose
A. Lactate, pyruvate, glycerol, glucogenic amino acid C-skeletons, odd-chain fatty acids, branched-chain fatty acids
B. To be utilized to do this they must 1st be converted into OAA, the starting material for this process (exception is glycerol; it enters as DHAP)
Gluconeogenic Building Blocks pt2
Substances that are broken down directly into acetyl-CoA CANNOT be used to make glucose
A. This includes Leu & Lys (ketogenic AA) , even- chain fatty acids, & ketone bodies
B. There is no pathway for converting acetyl- CoA into OAA
1. Remember C atoms entering TCA cycle in form of acetyl-CoA are lost as CO2
Lactate in Gluconeogenesis
I. Remember it was discussed that converting glucose into lactate was a huge waste of G, but that it is not
A. This is because of interorgan process called Cori cycle that occurs between liver & skeletal muscles
Cori cycle pt1
During strenuous physical activity, skeletal muscles switch to anaerobic metabolism to meet rapid ATP demands of cell
A. Causes buildup of lactate in skeletal muscles
II. Lactate is released from skeletal muscles into blood, where it is transported to liver
Cori cycle pt2
I. In liver, lactate dehydrogenase catalyzes its reverse reaction by
converting lactate into pyruvateA. This pyruvate feeds into gluconeogenesis, which leads to production of new glucose
B. This new glucose can be stored as liver glycogen, or it can be released back into blood for use by skeletal muscles
II. Gluconeogenesis requires G (hydrolysis of 4 ATP & 2 GTP)
A. Supplied by aerobic metabolism of fatty acids, which drives hepatic ATP synthesis
B. Represents net loss of 4 ATP ([6 NTP/Gluconeogenesis Used] – [2
ATP/Glycolysis Generated})1. Why humans cannot do anaerobic metabolism indefinitely