purine and pyridimine metabolism 1/2
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
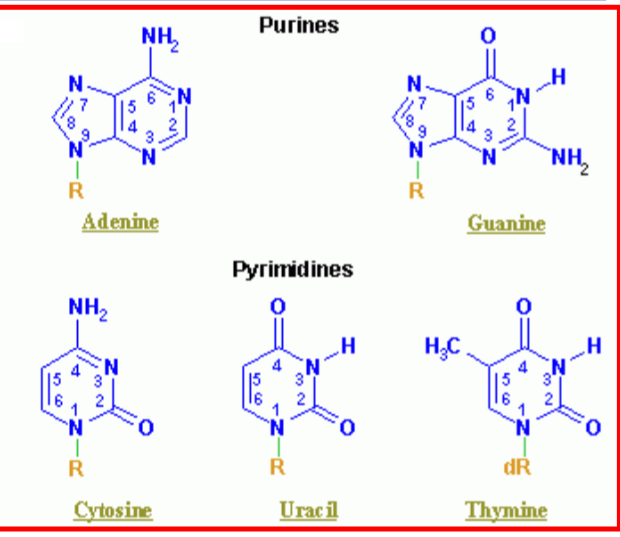
What are the two purines? What are the three pyridimines in genetic material? what is the difference between ribose and deoxyribose?
for deoxyribose, the second carbon has an -H instead of an OH (de-oxy)
what is a nucleoside?
ribose sugar group attached to a nitrogenous base
what is GTP mainly used for?
protein synthesis
what are UDP-glucose and UDP-glucoronic acid used for
they are used for synthesis of polysaccharides and oligosaccharides chains of glyco proteins
glucoronidation reaction-detoxification (bilirubin conjugation)
what is adenosine a major component of?
cofactors for oxidation reactions: NAD+/NADH; FAD/FADH2; NADP+/NADPH
What does AMP do?
component of CoA which is involved in
synthesis of bile acids
activation of acetate and Fatty acids for biosynthesis and catabolic reactions
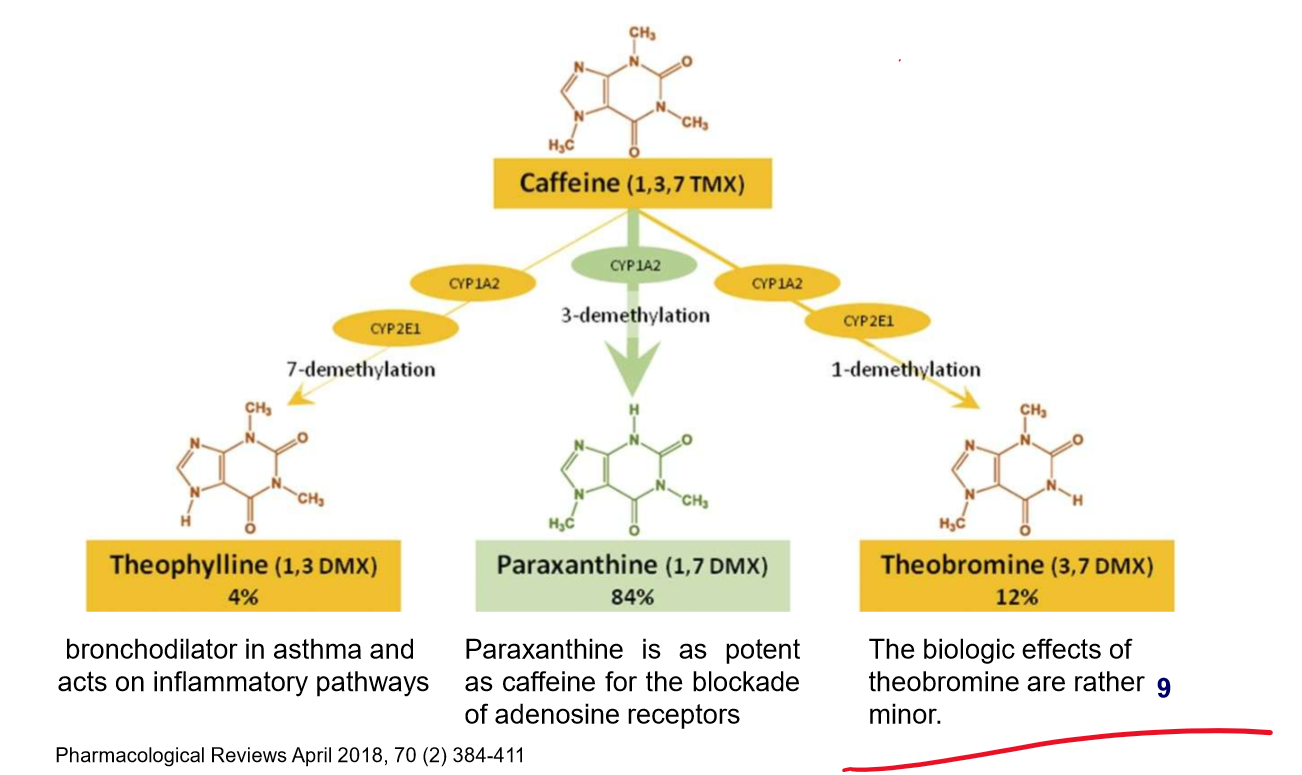
what medicine is caffeine a key element of? What is it an Antagonist of? What are its withdrawal symptoms?
key element of excedrin, Midol, migranal;
antagonist of adenosine receptors
withdrawal: headache, fatigue, and concentration problems
what three things can caffeine become and what are its functions?
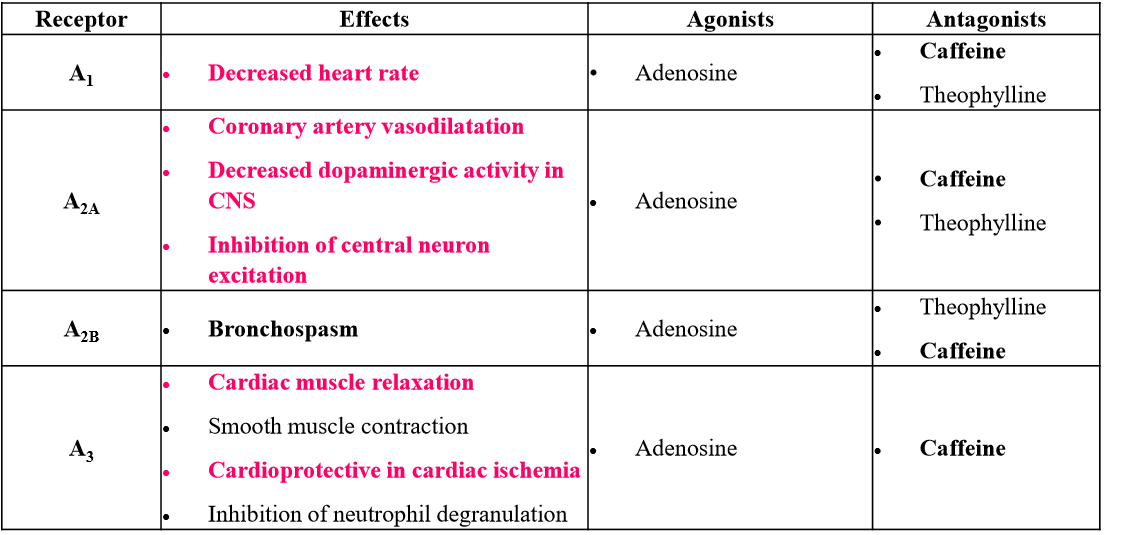
List out the adenosine receptors and their functions
talk about the signal transduction properties of adenosine
can stimulate or inhibit adenyl cyclase
secondary messengers: AMP (AMPK), cAMP (PKA), cGMP (PKG)
what is the difference between nucleoside and nucleotide
nucleoside: nitrogenous base linked to a pentose sugar; lacks a phosphate group
Nucleotide: a nucleoside + a phosphate group.
where do most of purine biosynthesis occur? how is it transported? what is the energy requirement for synthesis of one purine?
Most de novo synthesis occur in the liver but the brain could do some nucleotide synthesis as well;
nucleoside/bases are transported via RBCs
6-high energy bond for one purine; therefore, salvage pathways are used by many cells to recycle purine bases (convert free bases and nucleoside to nucleotide)
Draw out where each atom came from for the purine bases? How many molecules? How many precursors? How does synthesis occur?
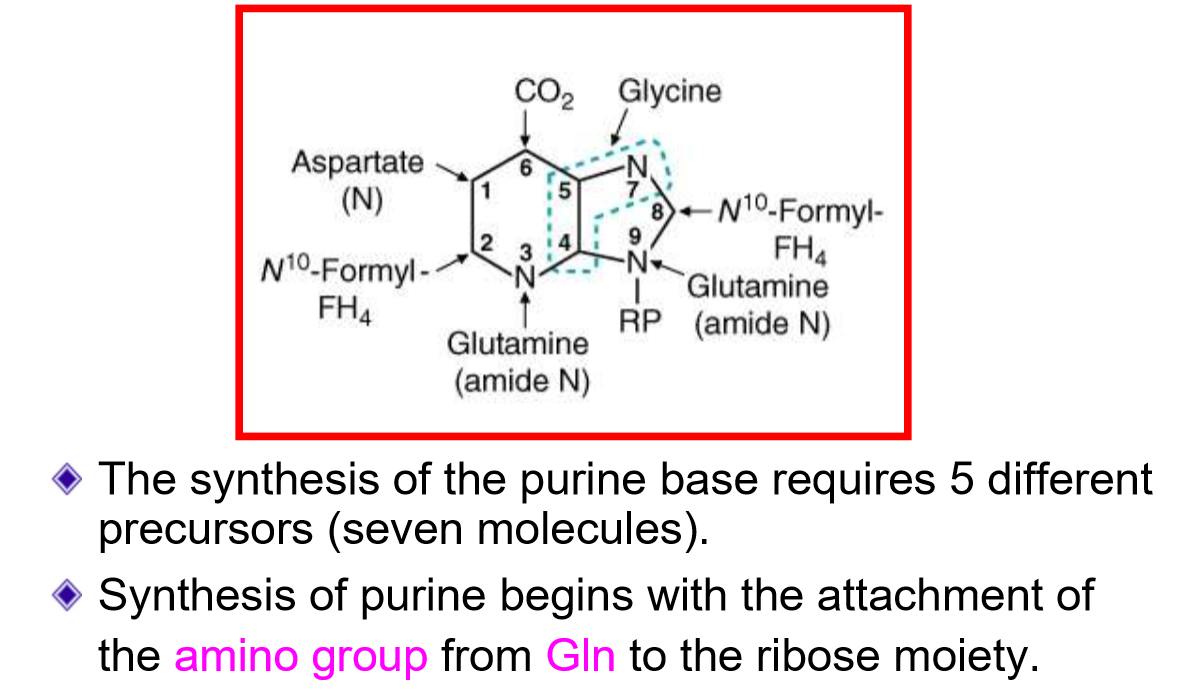
where are purines built from? What is the source of this? Describe this reaction? What is the limiting factor to this reaction?
built from activated form of ribose (5-phosphoribosyl 1- pyrophosphate (PRPP)
catalyzed by PRPP synthetase
ATP→ AMP
Limiting factor: low [ribose-5-phosphate]
Ribose-5-phosphate came from the PPP pathway
what is the first purine nucleotide created? describe this molecule and the reaction that created it. In this reaction, what happens if there is folate deficiency?
IMP (inosine monophosphate);
base = hypoxanthine
can be converted to either AMP or GMP
Reaction:
ten steps
uses 4 ATP
two N10- formyl-EH4 are used as donors of carbons
requires folate (B9) and cobalamin (B12) as recyling of folate
deficiency in folate = impair cell division = megaloblastic anemia
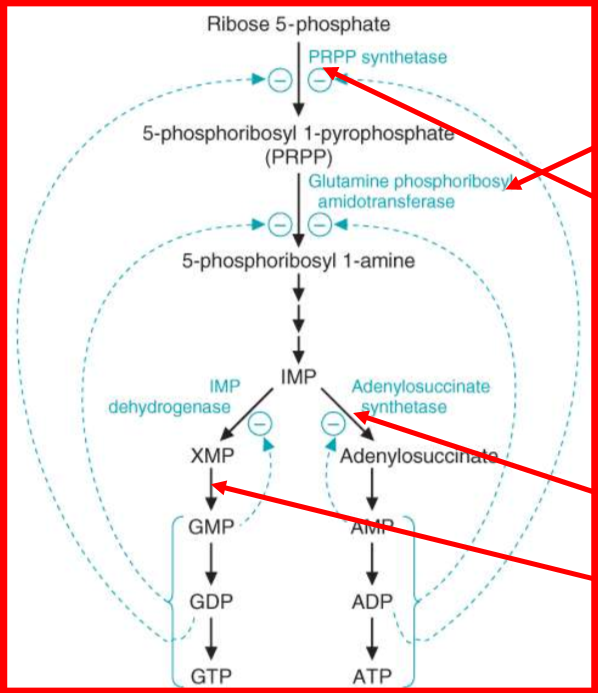
what is the commited step of purine biosynthesis. Describe this reaction and how it is regulated
PRPP —> 5-phosphoribosylamine
catalyzed by glutamine phosphoribosyl amidotransferase (GPA)
Glutamine → glutamate; H2O → 2PPi
regulated by: intracellular [PRPP] and [ GLN]; usual levels are far below GPA’s Km
increase in [] = increase in de novo synthesis
describe the synthesis of Adenosine Monophosphate
Aspartate + IMP → adenylosuccinate
catalyzed by adenylosuccinate synthetase
requires GTP
then, adenlosuccinate → AMP plus fumerate
catalyzed by adenylosuccinase
describe the synthesis of Guanosine Monophospahte
IMP → XMP (xanthosine monophosphate)
oxidized by IMP dehydrogenase
NAD+ + H2O → NADH + H+
Then, XMP → GMP
catalyzed by GMP synthetase
ATP + Gln → Glutamante + AMP
what is the amino group of AMP? GMP?
AMP: Asp
GMP: Gln
Describe how AMP and GMP become ATP and GTP?
nucleoside monophosphate Kinase: makes ADP and GDP
Nucleoside diphospahte kinase: makes ATP and GTP
describe the regulation of purine synthesis
what is the salvage pathway? why is it beneficial? what is the key enzyme? Why is this pathway important for some cell?
the salvage pathway interconnects free bases, nucleoside, and nucleotides
It is beneficial because it is energetically favorable and reduces levels of purine bases and nucleosides that could inhibit other metabolic reactions
key enzyme = hypoxanthine-guanine phosphoribosytransferase (HGPRT)
this is important because salvage of bases is a major form of nucleotide generation for lymphocytes!
Draw out the purine salvage pathways
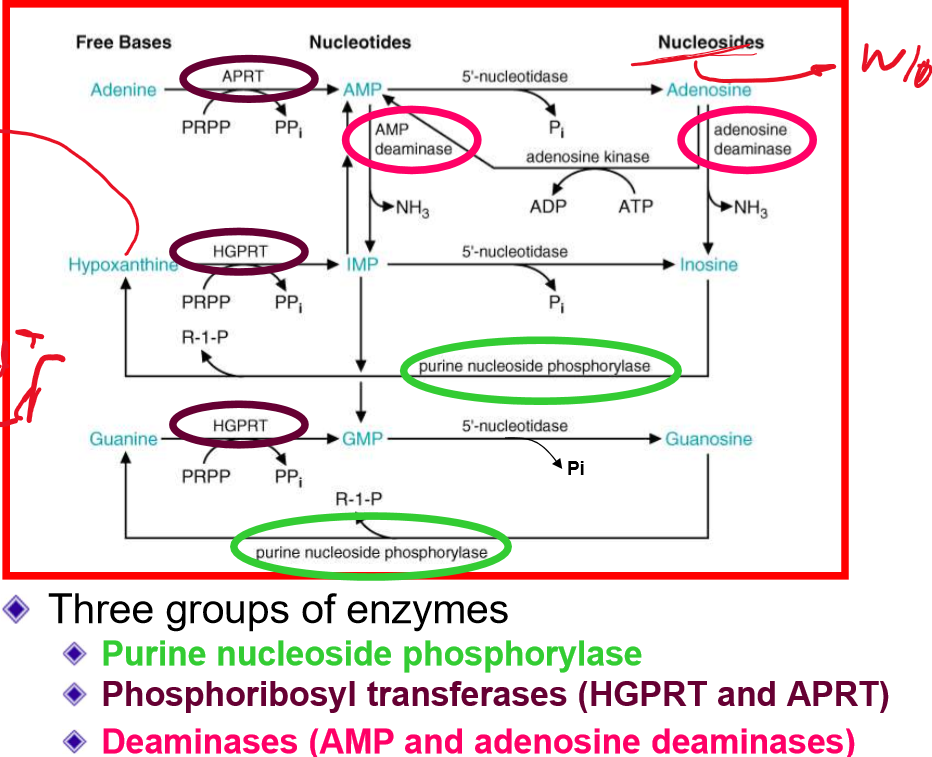
relating to the purine salvage pathway, deficiency of what enzyme leads to immunodeficiency?
deficiency of PNP (purine nucleside phosphorylase)
autosomal recessive
leads to T-cells toxicity and death
more sensitive to opportunistic infections
What is Lesch Nyhan Syndrome? explain the pathology, inheritance, symptoms and treatments
caused by deficient in HGPRT
since purine bases can’t be used → degradation → excessive uric acid
X-linked recessive
symptoms: mental retardation, self-mutilation (chew off fingers and lips), hyperuricemia (gout)
treatment: allopurinol (xanthine oxidase inhibitor)
What is ADA1 deficiency? explain its pathology
Adenosine deaminase deficiency
causes severe combined immunodeficiency disease (SCID)
deficiency = accumulation of deoxyadenosine → dATP → inhibits ribonucleotide diphosphate reductase
results in reduced generation of deoxyribonucleotides and impairs proliferation of lymphocytes
loss of immune system, no T/B cells
what is myokinase? What does deficiency of AMP deaminase cause?
during excercise, muscles can use myokinase to turn 2ADP → ATP + AMP;
result in muscle fatique (during excercise) , weakness, cramping, pain
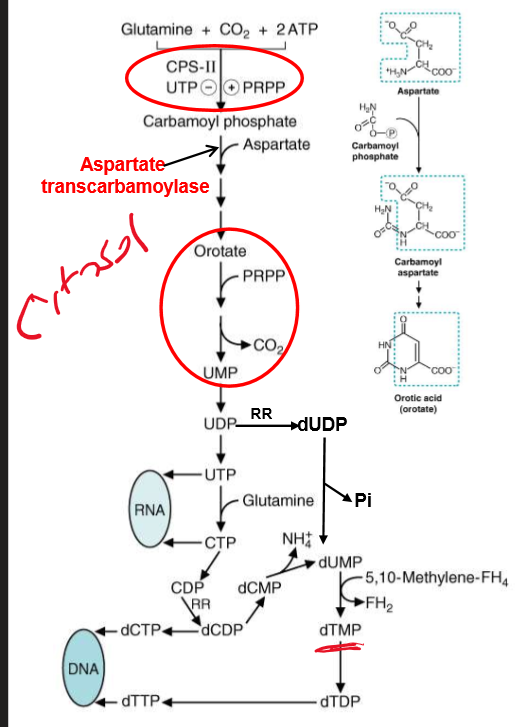
describe the synthesis of pyrimidines? What is CAD? what is carbamoyl phosphate derived from? How is CPSII different then CPSI?
based synthesized first
from Asp and carbamoyl phosphate
CAD = the first three enzyme of pathway rolled up into one peptide
Carbamoyl phosphate synthetase II, Asp transcarbamoylase, and dihydro-orotase
Carbamoyl phosphate derived from CO2 and Gln; II is different because uses Gln as source of nitrogen and occurs in cytosol.
how is pyrimidine synthesis regulated? how does folate deficiency relate to this?
regulation at CPSII
activated by PRPP, inhibited by UTP
phosphorylation by MAP kinase = more sensitive to PRPP, less sensitive by UTP
occurs as cell approach S-phase
folate deficiency result in anemia (macrocytic or macroblastic) bc of limited dTMP synthesis
draw out the general pathway for pyrmidine synthesis
draw out the step where orotate → UMP. Where are the two enzymes located? What are the consequences to deficiency of this and how do we treat this?
UMP synthase = polypeptide that contains both enzymes mentioned
Deficiency:
hereditary orotic acidura
accumulation of Orotic Acid
blocks pyrmidine synthesis = growth retardation, megaloblastic anemia
Treatment:
oral administration of Uridine
converts to UMP by bypassing metablic block
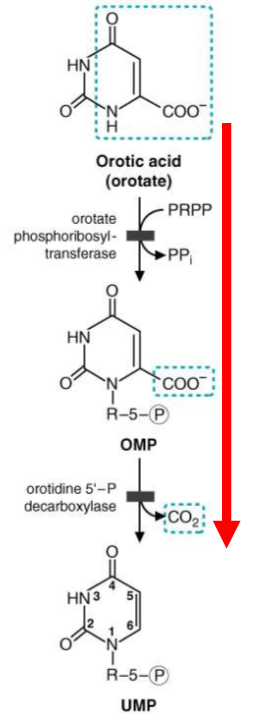
what is the second cause of orotic acidura?
deficiency in ornathine transcarbamoylase = accumulation of carbamoyl phosphate in mitochondria = leakage to cytosol; there pyrimidine production = orotic acidura
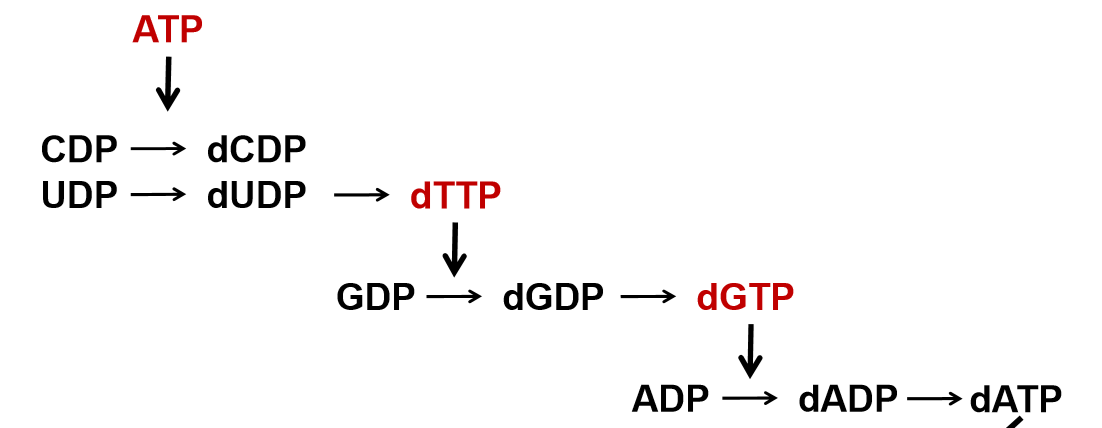
HOW is CTP produced? What are precursor to RNA Synthesis? How do we synthesize deoxyribonucleotides? how is dTTP produced?
CTP produced by addition of amino group from Gln to C4 of UTP
UTP and CTP = precursor to RNA
deoxyribonucleotides occur at the diphosphate level via Ribonucleotide reductase (RR)
CDP → dCDP; UDP → dUDP
dTTP is produced via methylation of dUMP
catalyzed by thy-midy-late synthase from N5,N10, methylene-THF
describe two cancer drugs
5-fluorouracil inhibits thymidylate synthase (no dTMP synthesis)
Metho- trexate inhibits dihydrofolate reductase (FH2 → 5,10, methylene-FH4
describe further the mechanism of methotrexate action. What is this drug used to treat?
inhibition of purine/pyrimidine synthesis
reduces antigen-dependent T-cell proliferation
suppress inflammation via adenosine release
used to treat;
cancer
psoriasis
rheumatoid arthiritis
systemic lupus erythematosus
Skin/muslce inflammation
Draw out how ribonucleotide reductase transform NDP to dNDP. Describe the two sites of RR
one site is for the alosteric regulator
ATP = activate
dATP = deactivate
second site = substrate specificity
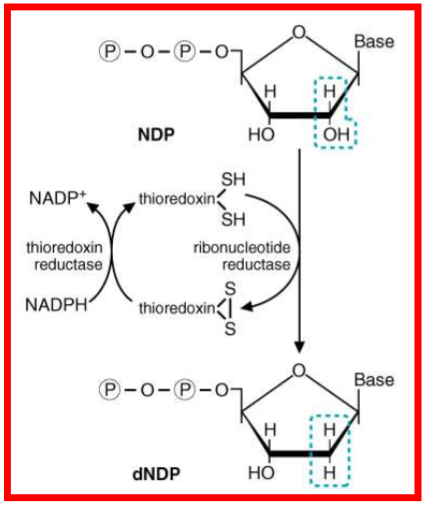
describe further how RR is regulated
draw out degradation of purine bases to uric acid; what does xanthine oxidase/dehydrogenase contain? What is the oxidase responsible for?
contains 2 molybdenum atoms
oxidase is responsible for oxidative damage to tissues during reperfusion
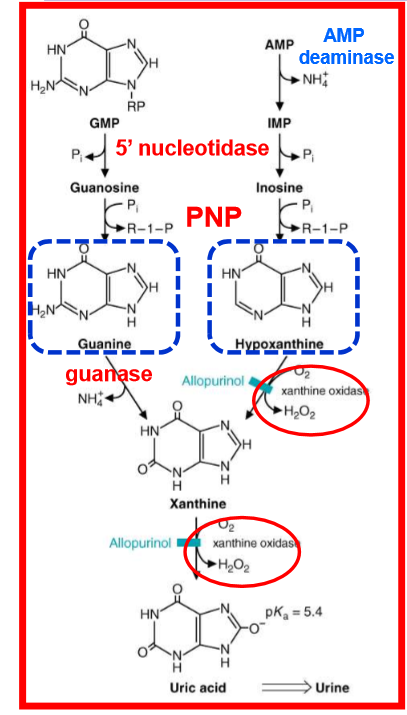
what is the final product of purine degradation? What is special at this? For this molecule, what happens at physiological pH?
uric acid; special becasue half of anti-oxidant capacity of blood plasma comes from this
at physiological pH, creates urate
Is urate soluble? what is hyperuricemia? What is the consequence of hyperuricemia?
no, normal urate [] very close to solubility constant
hyperuricemia: increased [urate] = formation and deposition of urate crystal in tissues and joint
describe factors contributing to hyperuricemia
gender (plasma uric acid is higher in males)
obesity
diet (high protein diet → rich nucleic acid and high alcohol consumption = higher levels of urate)
oxidation of alcohol = consumes ATP = increase Adenine nucleotide turnover
lactic acidosis
URAT1 exchanges lactic acid with reabsorption of uric acid
genetic factors (involved with renal urate transport system
What is gout? what are some disorder that causes overproduction of purines? treatments and prevention of gout?
metabolic arthritis; due to disorder of uric acid metabolism
associated with deposition of monosodium urate crystal
either underexcretion or too much production (primary gout) of uric acid
disorders that causes too much purines
PRPP synthetase overactivity
Glucose 6 phosphatase deficiency (vongierke)
Treatment
NSAIDS or injection of glucocorticoids
Prevention
allopurinol
limited consumption of alcohol (lactate production) and purine rich foods (meat, fish, spinach, and dry beans)
uricosuric drugs = increased excretion of uric acid from urine
what is xanthinuria?
disease where mutation in xanthine dehydrogenase gene or the molybdenum cofactor gene
causes catabolism of purine to stop at xanthine and hypoxanthine compounds
blood uric acid = low, high level of excretion of xanthine
can lead to formation of renal xanthine stones
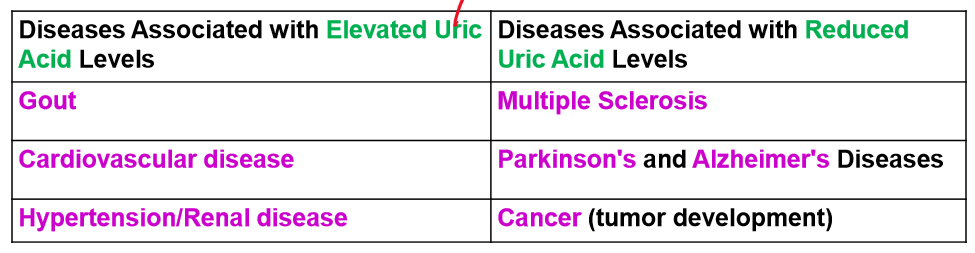
list out the diseases associated with elevated uric acid levels and ones with decreased levels
describe the mechanism of allopurinol. What is a complication of this
strucural analogue of hypoxanthine; means substrate for xanthine oxidase
converts to oxypurinol (inhibitor of xanthine oxidase)
decreased uric acid creation bc reduced purines can now be spread over three products: hypoxanthine, xanthine, and uric acid
Complication: rapid decrease in uric acid level = quick dissolution of urate crystal = trigger proinflammatory cytokine production and development of inflammation