B1 (Lifestyle, Health and Risk)
1/31
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Unicellular vs Multicellular organisms
Small unicellular organisms transfer substances through their cell membrane by diffusion, osmosis, or active transport.
Larger multicellular organisms require specialised exchange surfaces in the lungs and small intestine for example to move substances into the bloodstream.
Therefore small unicellular organisms are far more efficient at this.
Surface Area/Volume and diffusion distance
Surface Area/Volume: Which, simply, is the area on the outside of the organism, compared to its total volume.
Larger organisms simply don’t have enough surface area for substances to diffuse in quick enough. Mammals have a surface area/volume ratio which is too low for them to transfer substances by diffusion, so they require specialized exchange surfaces such as a circulatory system.
Mass transport
Mass transport (flow), is where liquids move down a pressure gradient.
Make up of blood
55% - Plasma (water, proteins, electrolytes, dissolved gases)
1% - Buffy coat (platelets and leukocytes)
45% - Erythrocytes
Blood vessels
Arteries:
Carry blood away from the heart to the body.
Have thick muscular and lots of elastic walls to deal with high pressure.
Have small passageways (lumen) for blood to flow.
Contains blood which is high in pressure.
Carries oxygenated blood (exceptions:pulmonary artery)

Veins:
Carry blood into the heart.
Have thin muscular walls.
Have wide lumen.
Contains blood that is low in pressure.
Have valves to prevent blood flowing backwards.
Carry deoxygenated blood (except pulmonary vein)

Capillaries:
Found in muscles and the lungs, for example.
Microscopic – one cell thick.
Very low blood pressure.
Where gas exchange takes place, oxygen can easily diffuse through the thin wall of a capillary.
Microvasculature
Water
The sharing of the electrons is uneven between the oxygen and hydrogen atoms.
The oxygen atom attracts the electrons more strongly than the hydrogen atoms, resulting in a weak negatively charged region on the oxygen atom (δ-) and a weak positively charged region on the hydrogen atoms (δ+).
4. This separation of charge due to the electrons in the covalent bonds being unevenly shared is called a dipole.
5. When a molecule has one end that is negatively charged and one end that is positively charged it is said to be a polar molecule.
6. Water is a polar molecule.
7. Hydrogen bonds form between the positive and negatively charged regions of water molecules because of the polar nature of water.
8. Hydrogen bonds are weak when they are few, so they are constantly breaking and reforming; this means that water molecules flow past each other in a liquid state.
9. Hydrogen bonds contribute to the many properties water molecules have that make them so important to living organisms.
Cohesion and Adhesion
Cohesion: Hydrogen bonds between water molecules allow for strong cohesion between water molecules. Cohesion is the attraction of water molecules to each other.
Adhesion: Water is also able to hydrogen bond to other molecules; this is known as adhesion.
Solvency
Due to the polar nature of water, water has a positive and negatively charged area. Negative ions, such as chlorine will form hydrogen bonds with the hydrogens of water. In the case of sodium and oxygen, electrostatic attraction would exist due to the differences in charge.
Other properties of water
Surface Tension
Colour
Density
State at room temp
Latent Heat
Specific Heat Capacity
The heart
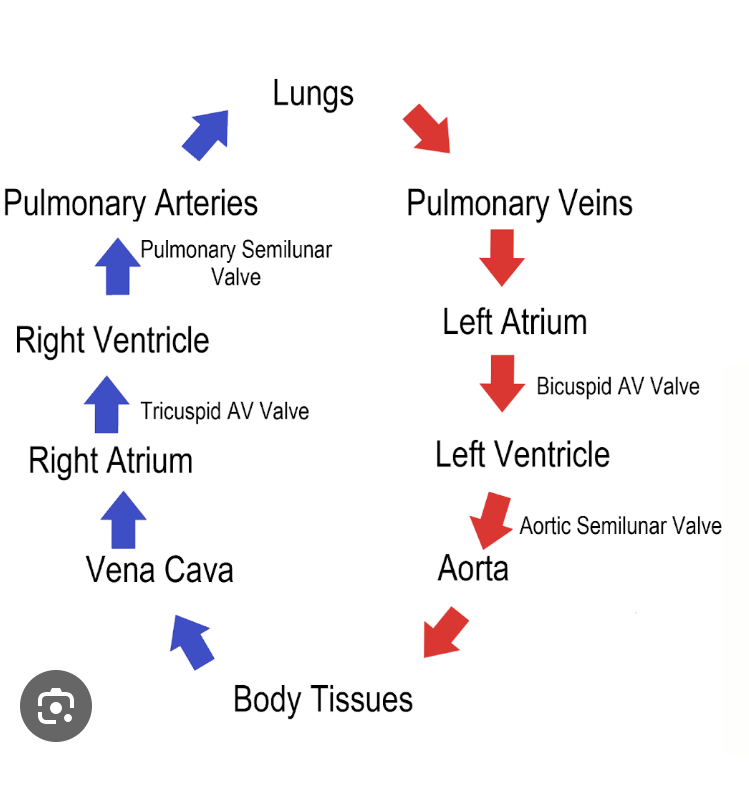
Deoxygenated blood is to the lungs and the left side pumps oxygenated blood to the rest of the body.
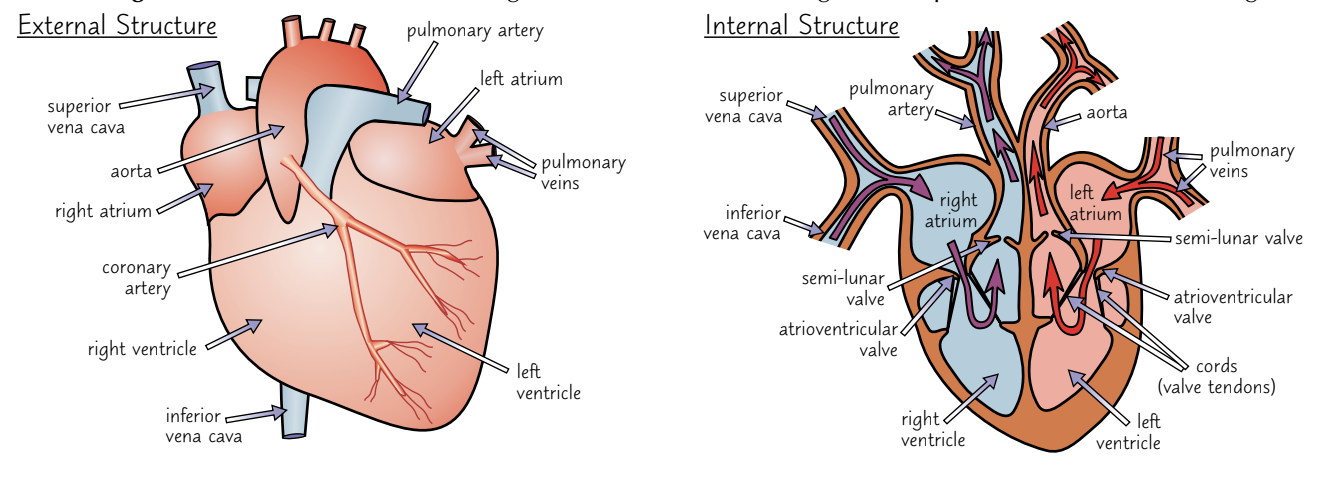
The left ventricle of the heart has thicker, more muscular walls than the right ventricle because it needs to contract powerfully to pump blood all the way around the body. The right side only needs to get blood to the lungs, which are nearby.
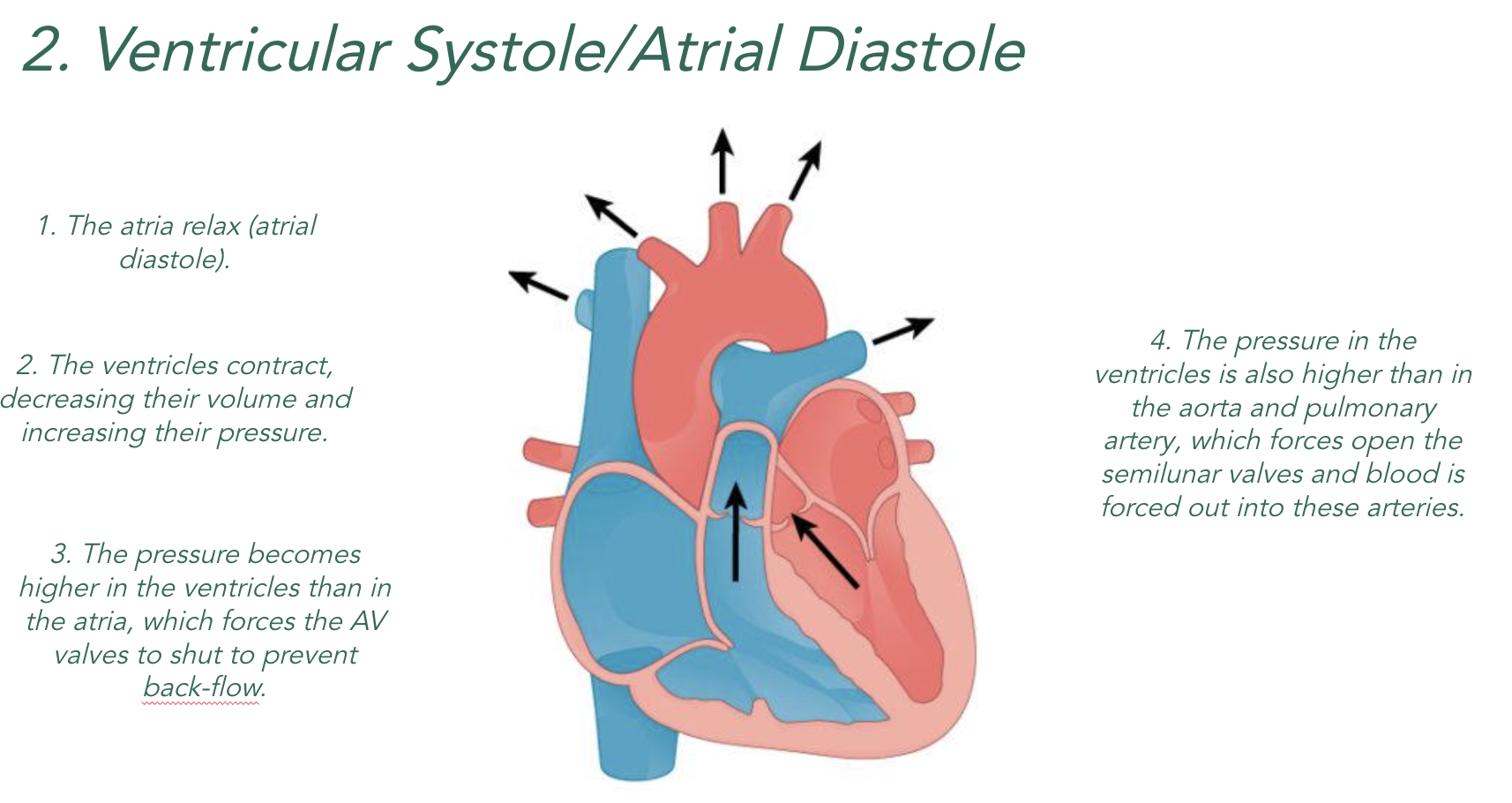
2) The ventricles have thicker walls than the atria because they have to push blood out of the heart whereas the atria just need to push blood a short distance into the ventricles.
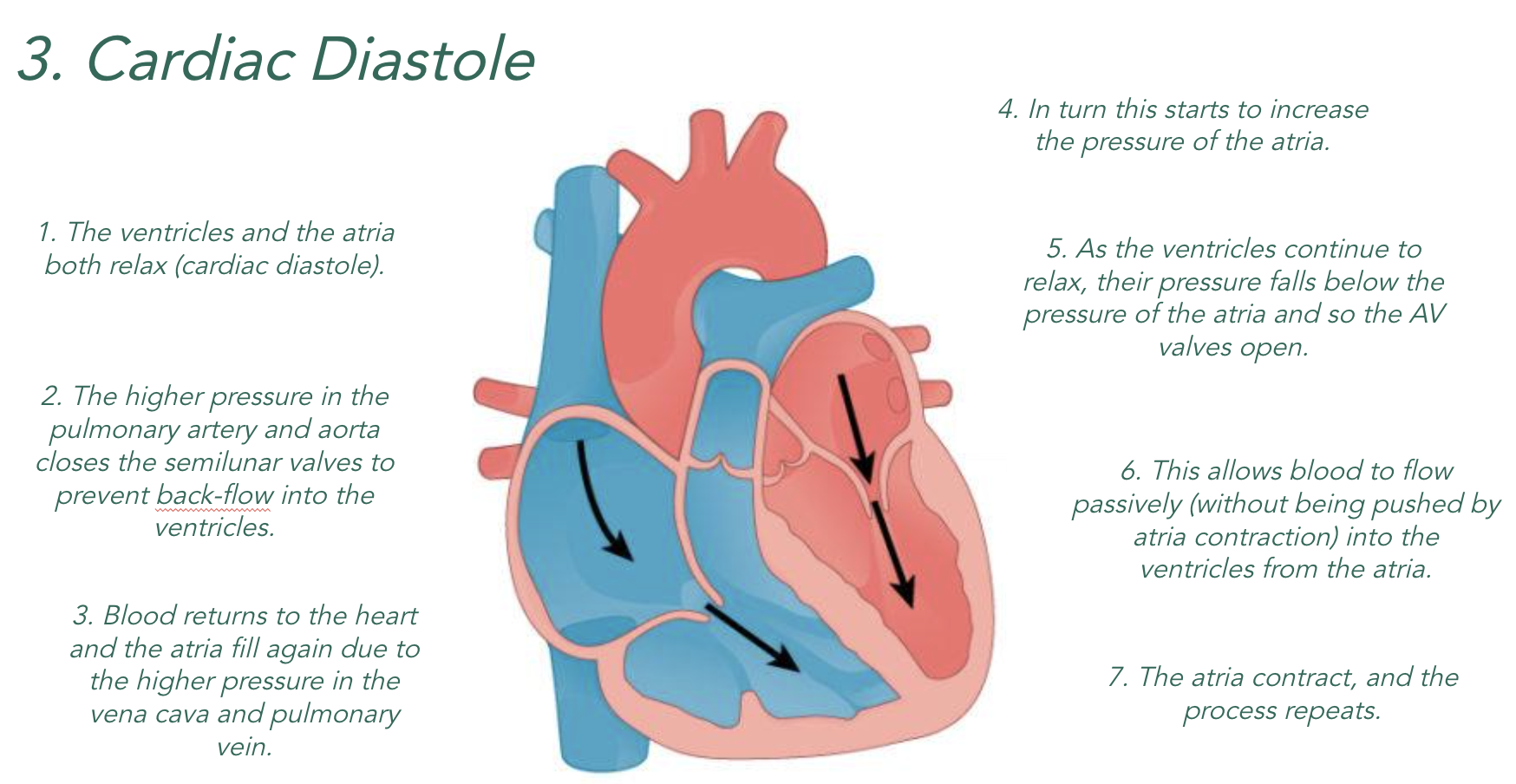
3)Valves maintain the correct pressure in the chambers of the heart. The valves open when the pressure of blood behind them is greater than the pressure in front and vice versa.
4) The atrioventricular (AV) valves link the atria to the ventricles and stop blood flowing back into the atria when the ventricles contract. Cords attach the atrioventricular valves to the ventricles to stop them from being forced up into the atria or flipping inside out when the ventricles contract.
5) The semi-lunar (SL) valves link the ventricles to the pulmonary artery and aorta and stop blood flowing back into the heart after the ventricles contract.
Blood flow around the body
More about the structure of the heart
•Covered by tough membrane = pericardium
•Encloses the pericardial fluid (lubricates the hearts movement against the membrane)
•Atria are thin-walled because they pump blood a short distance to the ventricles.
•Ventricles have thick walls because they need to pump blood a longer distance to either lungs or rest of body.
•Left ventricle much thicker than right
•The left atrioventricular valve (also known as bicuspid or mitral valve) is formed of two cup-shaped flaps.
•The right atrioventricular valve (or tricuspid valve) formed of three cup-shaped flaps.
•Tendons (heart strings) attach the valves to the ventricular walls preventing the valves turning inside out.
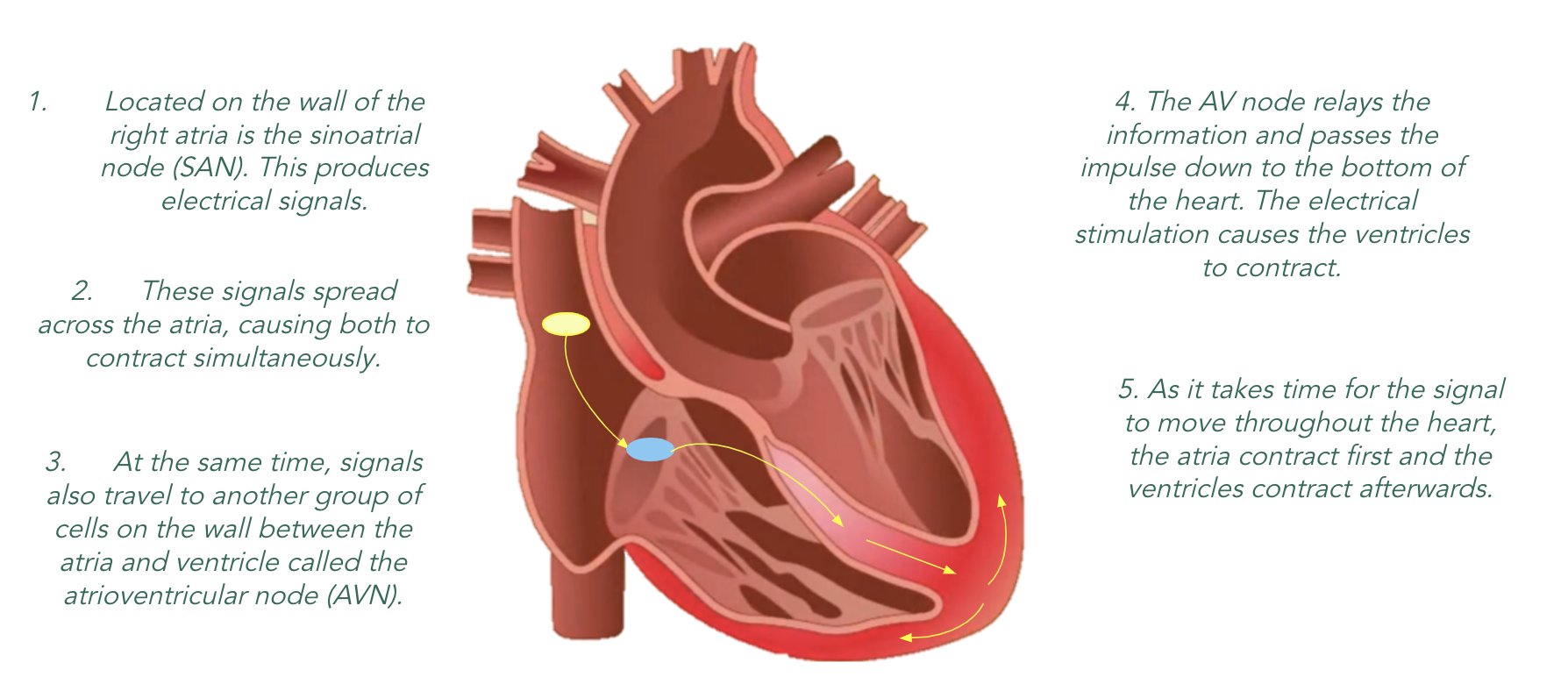
Cardiac cycle
•One cardiac cycle consists of the atria and then the ventricles contracting so that the blood that has entered the heart is pumped out.This occurs about 70 times every minute
The periods of contraction are called systole.
The periods of relaxation are called diastole.
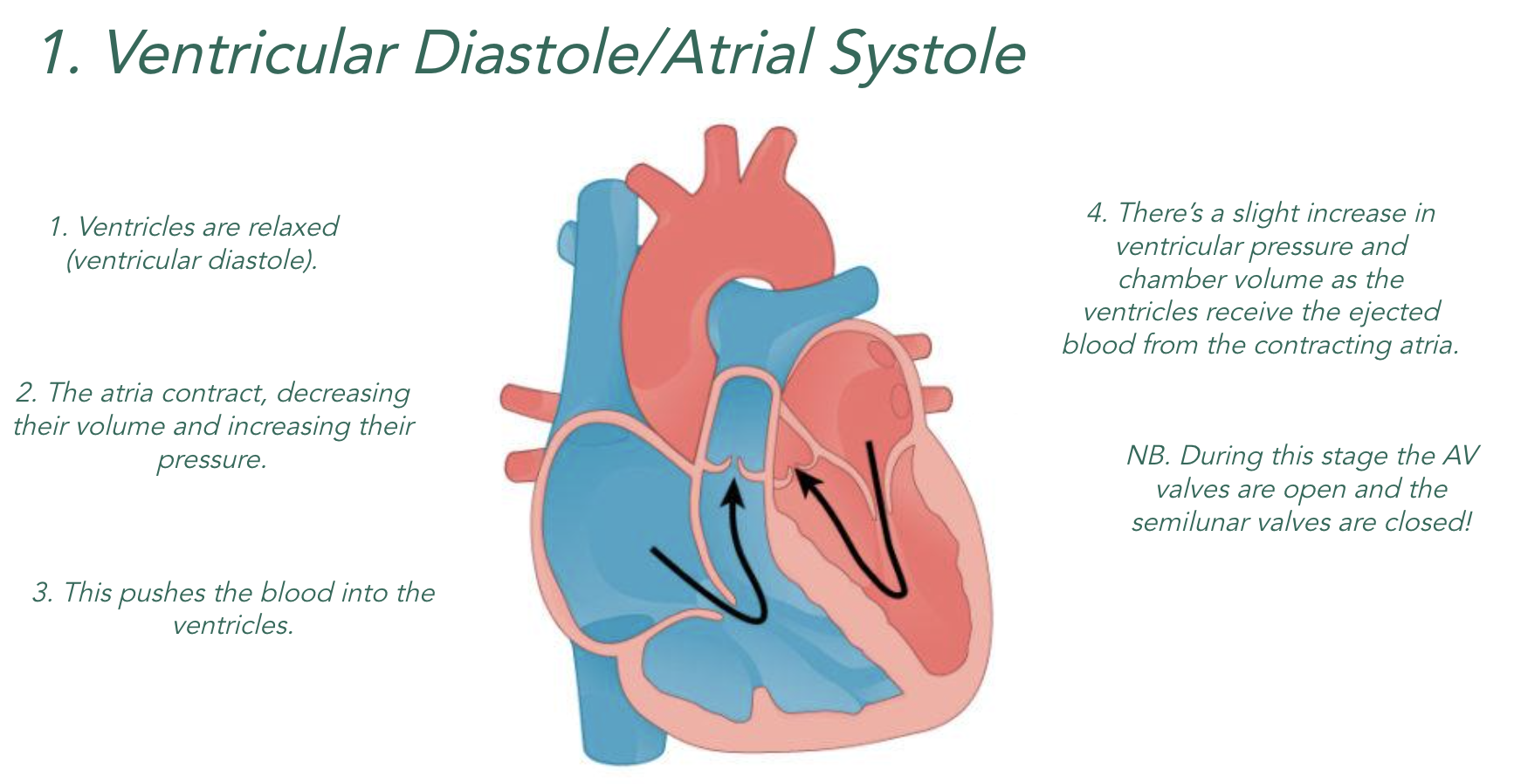
When the chambers of the heart contract (systole), they become smaller which means…
Lower Volume = Higher Pressure
When the chambers of the heart relax (diastole), they become bigger which means…
Higher Volume = Lower Pressure
Carbohydrates
Carbohydrates are found in starchy food, e.g., potatoes, pasta, rice and bread.
Consist of carbon, hydrogen and oxygen only
Their function is to be a store of energy.
Most carbohydrates are large, complex molecules (polymers) made of long chains on monosaccharides (monomers).
Monosaccharides are also carbohydrates.
Glucose
Can be stored in our bodies as glycogen (a starch).
Transported throughout the body in our blood.
Levels are controlled by the pancreas and the liver.
A reactant of aerobic and anaerobic respiration.
Glucose’s structure is related to its function as the main energy source in animals and plants.
Its structure makes it soluble so it can be easily transported, and its chemical bonds contain lots of energy.
Monosaccarides
Taste sweet when dissolved in water.
Will have the same number of oxygen atoms to carbon, e.g. C6H12O6
White crystalline solids.
Have the general formula (CH2O)n.
Other examples include; fructose, galactose, ribose, deoxyribose.
Disaccarides
Disaccharides form when a condensation reactions occurs between two monosaccharides. In a condensation reaction, water is removed. A glycosidic bond forms whereby an oxygen links the two monosaccharides.
They are formed by condensation reactions between two monosaccharides.
They are soluble (though not as soluble as monosaccharides).
Their chemical bonds store more energy than the bonds in monosaccharides.
The glycosidic bond in a disaccharide will have numbers…
Disaccharides can be broken apart using hydrolysis reactions.
Polysaccarides
A polysaccharide is formed when more than two monosaccharides join together.
Starch (main energy source in plants):
1) Cells get energy from glucose. Plants store excess glucose as starch (when a plant needs more glucose for energy it breaks down starch to release the glucose).
2) Starch is a mixture of two polysaccharides of alpha-glucose: amylose and amylopectin:
• Amylose — a long, unbranched chain of glucose joined together with 1-4 glycosidic bonds. The angles of the glycosidic bonds give it a coiled structure, almost like a cylinder. This makes it compact, so it’s really good for storage because you can fit more in to a small space.
• Amylopectin — a long, branched chain of glucose that contains 1-4 and 1-6 glycosidic bonds. Its side branches allow the enzymes that break down the molecule to get at the glycosidic bonds easily. This means that the glucose can be released quickly.
3) Starch is also insoluble in water, so it doesn’t cause water to enter cells by osmosis, which would make them swell. This makes it good for storage. No osmotic effect.
Glycogen (main energy store in animals)
1) Animal cells get energy from glucose too. But animals store excess glucose as glycogen another polysaccharide of alpha-glucose.
Its structure is very similar to amylopectin (it has 1-4 and 1-6 glycosidic bonds), except that it has loads more side branches coming off it. Loads of branches means that stored glucose can be released quickly, which is important for energy release in animals.
It’s also a very compact molecule, so it’s good for storage.
Like starch, glycogen’s also insoluble in water, so it doesn’t cause cells to swell by osmosis.
It’s a large molecule, so it can store lots of energy.
Lipids
Lipids are a biomolecule group made of carbon, hydrogen, and oxygen atoms, that are mostly non-polar due to their hydrocarbon chain structures.
They have a variety of roles including:
Cell membrane structure – Phospholipids
Energy storage – More compact than glycogen
Water repellent (animal fur) – Hydrophobic
Hormone synthesis – Steroid hormones
Insulation – Visceral (around organ) and subcutaneous (under skin) fat
Triglycerides
The type of lipid we must know are triglycerides (also called triacylglycerols). This lipid can come in two forms; fats and oils.
Fats are solids at room temperature,
Oils are liquids at room temperature.
The backbone of these forms is a glycerol (type of alcohol) molecule. These forms the foundation for the structure of this typical lipid. It has the formulae C3H8O3.
A triglyceride is made of one molecule of glycerol with three fatty acids attached to it. Fatty acid molecules have long tails made of hydrocarbons (carbon chains with hydrogen atoms branching off). The tails are hydrophobic (water-repelling). These tails make lipids insoluble in water.
Fatty acids
Fatty acids are long chains consisting of:
Methyl group (-CH3) at one end
Hydrocarbon chain (Hydrogen and carbon)
Carboxyl group (-COOH) at the other end
The length of the hydrocarbon chain impacts the type of fatty acid and whether it is a fat or an oil.
All fatty acids have the same structure (-CH3, hydrocarbon chain, -COOH). The types of bonds in the hydrocarbon chain determines their type. There are two types:
Saturated – Single-chain with all carbon atoms in the hydrocarbon chain fully ‘saturated’ – This means that there are no double-bonds between the carbons. They are fats as they are solids at room temperature.
Unsaturated – Have at least one double-bond (carbon-carbon) in their structure. Mostly liquid at room temperature, therefore are oils.
If there is only one carbon-carbon double-bond, this is referred to as monounsaturated. If there are many, it is called polyunsaturated.
Trans and cis unstaurated
Within unsaturated fatty acids, whether mono- or poly-, the position of the hydrogen atoms at the carbon-carbon double bonds is important:
-Trans – This is when the hydrogens at the carbon-carbon double bond are on opposite sides. Linked to bad health as they don’t metabolize well.
-Cis – This when both hydrogens at the carbon-carbon double bond are on the same side. With moderate intake, these have been shown to be beneficial
Ester bonds
The hydroxyl (OH) groups on the glycerol molecule can form condensation reactions with the OH part of the carboxyl (COOH) on a fatty acid. These allows them to form ester bonds, the process of which is called esterification. It is important to say in the exam that, like in saccharides, water molecules are released (3H2O)
LDLs and HDLs
1) Cholesterol is a type of lipid that is made in the body.
2) Some is needed for the body to function normally.
3) Cholesterol needs to be attached to protein to be moved around, so the body forms lipoproteins - substances composed of both protein and lipid. There are two types of lipoprotein:
HIGH DENSITY LIPOPROTEINS (HDLs):
They are mainly protein.
They transport cholesterol from body tissues to the liver where it’s recycled or excreted.
Their function is to reduce total blood
cholesterol when the level is too high.
LOW DENSITY LIPOPROTEINS (LDLs):
They are mainly lipid.
They transport cholesterol from the liver to the blood, where it circulates until needed by cells.
Their function is to increase total blood
cholesterol when the level is too low.
High total blood cholesterol level (the level of HDL, LDL and other cholesterol) and high LDL level have both been linked to an increased risk of CVD. An increased cholesterol level is thought to increase atheroma formation.
Atherosclerosis
Atherosclerosis is a hardening of the arteries. A fatty plaque builds up and can continue until blood flow is restricted or blocked.
Atherosclerosis can occur mainly in a coronary artery (at the heart) or carotid arteries (at the neck)
atherosclerosis occurs in stages:
Damage to the endothelial lining
Inflammatory response
Atheroma on lining
Atherosclerosis can also cause an aneurysm (when weakened artery walls split open, leading to internal bleeding)
Stage 1
Stage 2
Stage 3
•There is damage to the endothelial cells of the artery
•Usually due to high blood pressure
•This damage brings about an inflammatory response and white blood cells migrate to the walls of the arteries
•These cells begin to accumulate cholesterol
•Fatty plaque builds up
•Calcium salts and fibrous tissue also build up
•Fibrous tissue hardens the wall of the artery leading to loss of elasticity
•Lumen narrowed , increasing blood pressure
•Self perpetuating – positive feedback – increased pressure causes more damage to the endothelial cells
Angina
With Angina plaque builds at coronary arteries, reducing blood flow to parts of the heart. This reduced blood leads to increased anaerobic respiration. A stent is often used to treat, however dilating drugs are mainly used.
Myocardial infarction
Myocardial Infarctions are caused by a blood clot as a result of atherosclerosis. Platelets begin the clotting process at plaque. Along with this the plaque may rupture, the cholesterol released will be acted upon by platelets. The clot that forms is called thrombosis.
Stroke
A stroke is in interruption of normal blood flow to the brain; via damage, clotting, or bleeding in blood vessels.
It may be caused by an atheroma or blood clot. In arterioles, the damage is less severe. However, a stroke as a result of a blocked primary artery can be very serious.
Blood clotting
This process of clotting from start to end is called hemostasis (clotting).
The initial response of platelet activation then leads to a sequence that prevents bleeding and begins to repair the damaged area.
Stage One – Initial Damage
A damaged vessel causes the release of serotonin, a neurotransmitter
This causes vasoconstriction at that vessel, reducing the blood flow at the site of damage.
Stage One – Platelet Activation
Collagen fibres from the vessel are now exposed.
Platelets are activated when in contact with collagen, changing shape and becoming adhesive.
They begin to stick and plug at the site.
Stage Two & Three – Clotting Cascade
Platelets cause the release of thromboplastin (a protein) that starts the sequence
This catalyzes (using Ca2+) prothrombin (an insoluble protein) into thrombin (soluble version)
Thrombin catalyzes fibrinogen to form fibrin. Fibrin acts as a mesh, covering and trapping the clot.
Risks in CVD
It is rare when a single cause is linked to a single illness. We know that many cardiovascular diseases (CVD) are multifactorial diseases (influenced by multiple factors).
When viewing these types of diseases it is important to look at the risk factors that link to a multifactorial disease.
Risk factors increase the probability of someone having a disease due to a factor(s) when compared to a group without the disease.
Multiples risk factors increase probability (e.g. high cholesterol and a family history of multiple heart failures)
For risk factors related to CVD longitudinal studies are performed. These are studies where participants are tracked over a long time period to observe similarities and differences in lifestyle and environmental factors (e.g. those with lack of physical activity appear to be more likely to have pre-mature mortality)
From there a hypothesis is formed and the sample selection and size is chosen.
The hypothesis formed is used to fuel an investigative study (e.g. an increase in smoking leads to increased risk of CAD (Coronary Artery Disease)).
Treating CVD
Antihypertensives:
•Drugs used to lower blood pressure
•Reduces damage to endothelium of artery walls
•Reduced risk of inflammatory response (and so plaques and CVD)
•EG Beta blockers - block receptors on the motor neuron leading to the heart muscle, so heart doesn’t speed up & blood pressure stays low
•Vasodilators – widen arterioles & lower blood pressure
•Diuretics – increase water loss, so reduce blood volume & pressure
•Risks –palpitations & drowsiness
Statins:
•Reduce the amount of cholesterol in the blood
•Plant statins
•Reduce the absorption of cholesterol in the small intestine
•Statins
•Reduce LDL cholesterol levels in the blood
•Inhibit an enzyme which makes cholesterol in the liver
•Less likely that the fatty plaque will develop
pros:•Lower cholesterol, lower chance of fatty plaques, lower CVD & CHD risk •Effective •Few side effects •Serious side effects very unlikely •Risks of CVD outweigh the risks of statins •Relatively cheap (off patent)
cons:•Side effects •Muscle fatigue •Liver damage (rare) •Type 2 diabetes (needs monitoring) •Cost to the NHS (but less than treating CVD) •Does not encourage lifestyle change & there are other risk factors of a poor diet & lack of exercise •Overall risks of CVD outweigh the risks of statins
Anticoagulants:
•Prevent blood clots forming
•Usually inhibit the production of fibrin
•So less likely that artery will get blocked
•Reduce chance of stroke and myocardial infarction
•Risks – excessive bleeding & swelling of tissues
Platelet inhibitors:
•Reduce the growth of blood clots that have already formed
•Make platelets less sticky
•So less likely that artery will get blocked
•Reduce chance of stroke and myocardial infarction