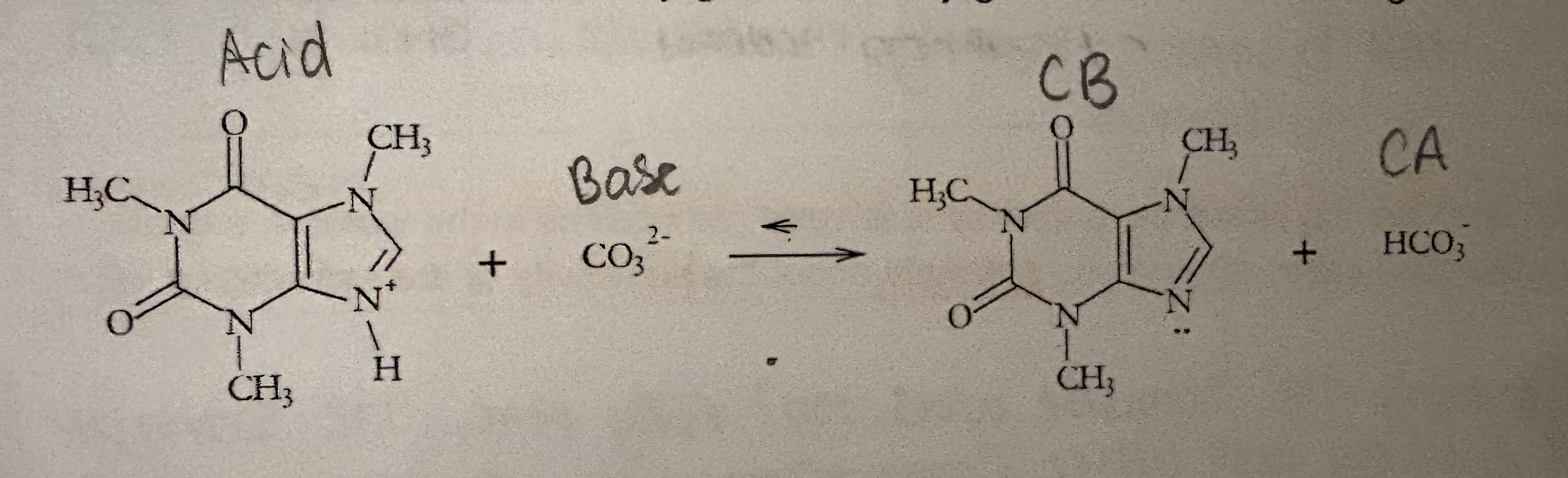Caffeine Lab Super Chem
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
17 Terms
Overall goal
Try to extract pure caffeine
2 safety precautions
Goggles and wash hands thoroughly with soap and water before leaving the lab
Miscibility
Ability of substances to mix in any proportion to form a homogenous mixture, meaning they dissolve completely in one another
Emulsions
A mixture of two or more liquids that are normally immiscible, like oil and water
Bumping
Sudden, violent boiling of a liquid that has become superheated (controlled by boiling stones)
Tea contains 5% caffeine by weight. What mass of caffeine should have been in your tea? (16g of Tea)
0.8g
What are the two ways Na2CO3 aids the extraction of caffeine?
Converts caffeine into its non-ionic form which increases the solubility in Ethyl Acetate and decreases solubility in water.
Increases solubility of tannins and Saponins in water while decreasing solubility in ethyl acetate (less polar than water)
Density
The property of an organic solvent that determines the position of the immiscible layer it forms with water
[Less dense on top, Most dense on bottom]
why are two extractions of ethyl acetate is more effective than one
2 extractions pull out more caffeine from the water layer
Boiling stones
To control bumping/boiling, makes it a steady and even process
[TLC analysis may reveal the presence of several compounds in this isolated sample.]
What would this observation allow you to conclude ab the selectivity of the extraction process?
It means the sample was not fully pure, the sample most likely has other components like tannins and saponins.
How to identify the presence on caffeine?
Using UV light to determine how far the substance traveled. By collecting the distance the component moved compared to the solvent (Rf value), for the caffeine extract & standard, we were able to determine how much caffeine we extracted.
Sodium Sulfate (Na2SO4)
used to absorb excess water from the organic layer.
What if the Na2CO3is not added in the beginning? Would the experiment work?
NO, we would have collected other compounds like saponins and tannins along with our caffeine, which would also stay more soluble in water
Why is NaCl added before putting the solution into the separatory funnel?
NaCl decreases the Miscibility/unwanted emulsions of aqueous and organic layer
Determine A, CA, CB, & B
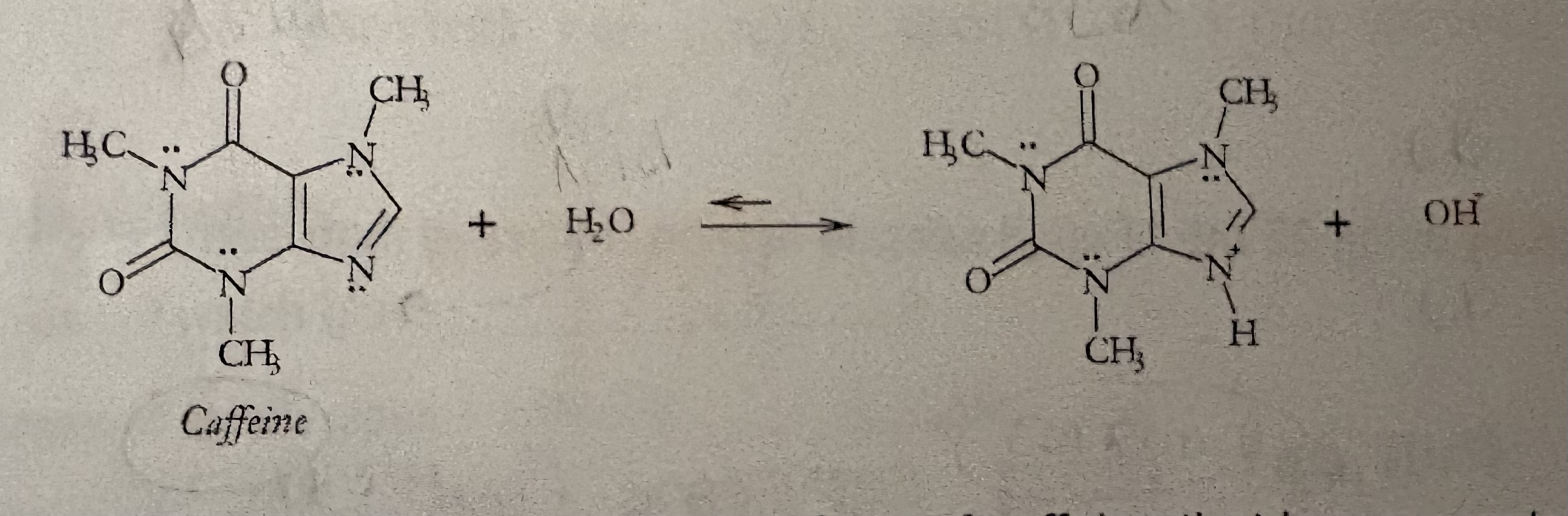
What is the form of Caffeine that is more water soluble
what is the form of Caffeine that is more soluble in Ethyl acetate
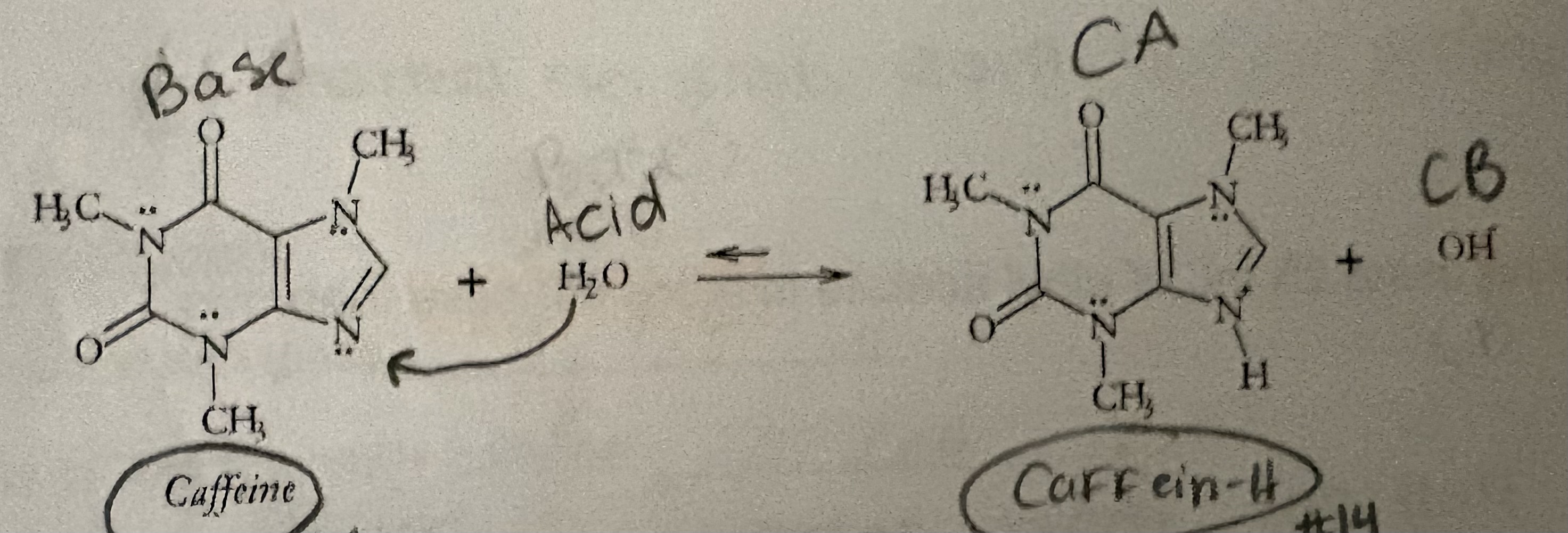
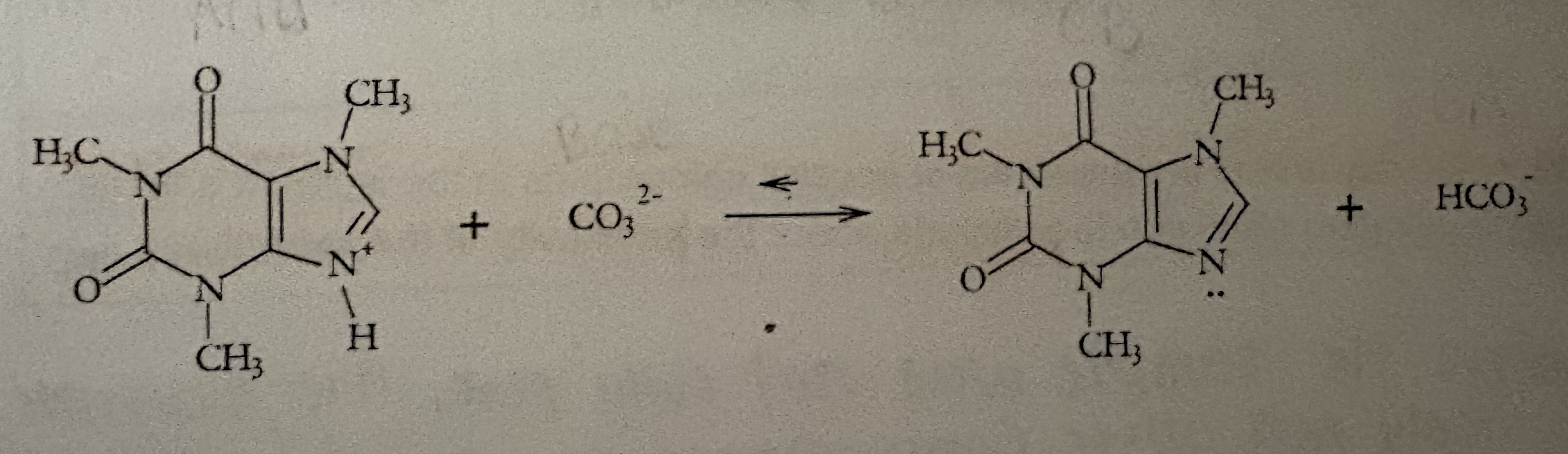
Determine A, B, CA, & CB