B1.1: Carbohydrates and Lipids
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Four main categories of molecules in living organisms
Carbohydrates
Lipids
Nucleic Acids
Proteins
What do the four main categories of molecules in living organisms all share in common?
Carbon, hence why life is often known as ‘carbon-based’
Carbon properties
Four valence electrons, which means it can covalently bond four times with other elements/compounds/molecules
Can form covalent bonds
Can form double bonds and bonds with itself
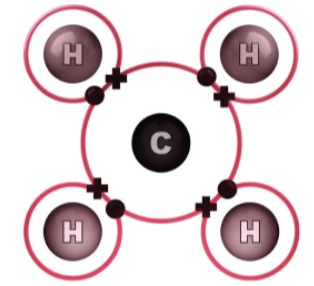
Other elements associated with the molecules in living organisms
memorise each of their valence electrons
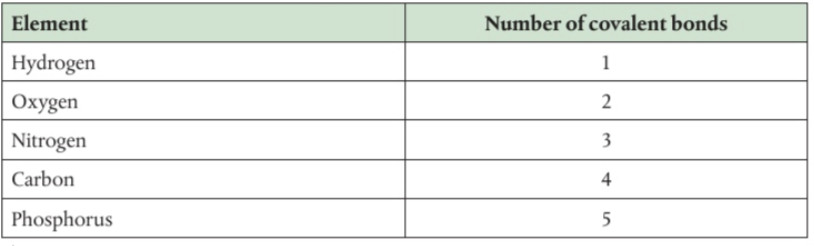
Functional groups
specific groupings of atoms within molecules that have their own characteristic properties, regardless of the other atoms present in a molecule
Common Functional groups
OH- hydroxyl or alcohol
NH2- amino or amine
COOH- carboxyl
H2PO4- phosphate
Categories and examples of four biochemically important molecuels
Category | Subcategory | Example Molecules |
|---|---|---|
Carbohydrates | Monosaccharides | Glucose, galactose, fructose, ribose |
Disaccharides | Maltose, lactose, sucrose | |
Polysaccharides | Starch, glycogen, cellulose, chitin | |
Proteins | Enzymes, antibodies, peptide hormones | |
Lipids | Triglycerides | Fat stored in adipose cells |
Phospholipids | Lipids forming a bilayer in cell membrane | |
Steroids | Some hormones | |
Nucleic Acids | Nucleotides | DNA, RNA, ATP (adenosine triphosphate) |
Macromolecules
a very large molecule important to biological processes, which are made of smaller molecules called monomers.
When you digest food, many of the molecules of the food are in the form of these molecules.
Monomers
atoms or small molecules that bond together to form more complex structures such as polymers.
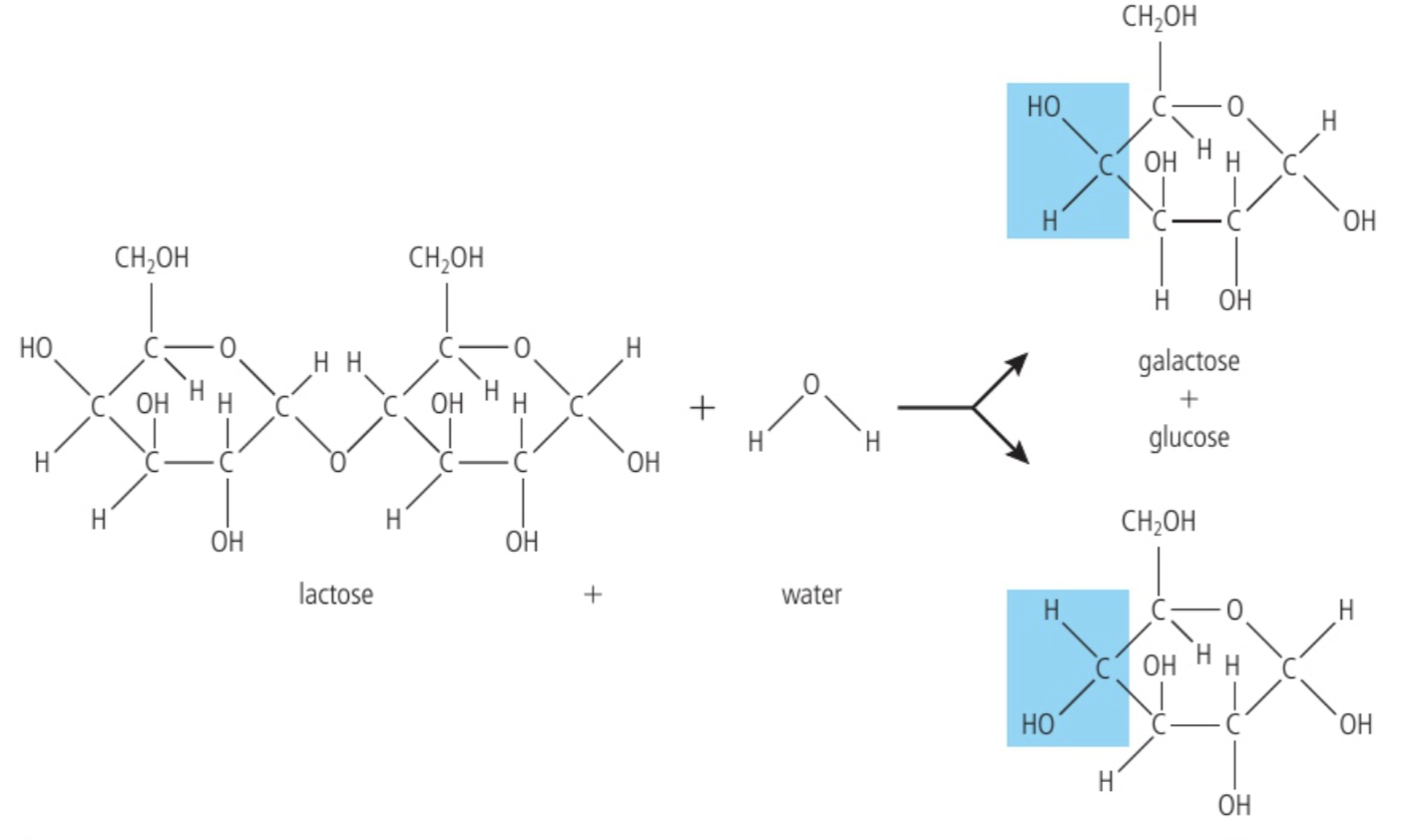
Hydrolysis
Digestion breaks down macromolecules through this process of chemical reactions. It breaks down covalent bonds between monomers.
once the monomers have been broken down, they are then a suitable size to be absorbed into the bloodstream and circulated into body cells
Macromolecules and their monomer subcomponents
Macromolecule | Monomer |
|---|---|
Carbohydrates | Monosaccharides |
Lipids | Glycerol, fatty acids, phosphate groups |
Proteins (polypeptides) | Amino Acids |
Nucleic Acids | Nucleotides |
Condensation reactions
after entering cells, the monomers recombine (built up) to form macromolecules once again by forming covalent bonds through this reaction.
Example of hydrolysis and condensation reactions
You eat a taco containing beef, a source of protein.
Hydrolysis reactions occur in the digestive system, resulting in amino acids.
Amino acids are absorbed into the blood and taken to body cells.
DNA in a body cell directs specific condensation reactions to produce a specific protein from the amino acids.
Condensation & Hydrolysis reactions and water
Condensation reaction: creates water molecule
Hydrolysis: breaks water molecule into two separate components, which then forms into two smaller molecules
Linking monomers to polymers
The "R" notation in Figure 2 indicates that these two amino acids could be any of the 20 different possibilities. Notice that a portion of the carboxyl group of one amino acid becomes oriented near the amine group of the other amino acid. Stress is placed on the -OH from one amino acid and the H of the other. This results in the covalent bonds breaking. The released -OH (hydroxide ion) and H (hydrogen ion) combine to form a water molecule. The location where the -OH and H were released still contains a pair of electrons that form a new covalent bond. Whenever this occurs between two amino acids, the new covalent bond is called a peptide bond. The reaction is catalysed by an enzyme.
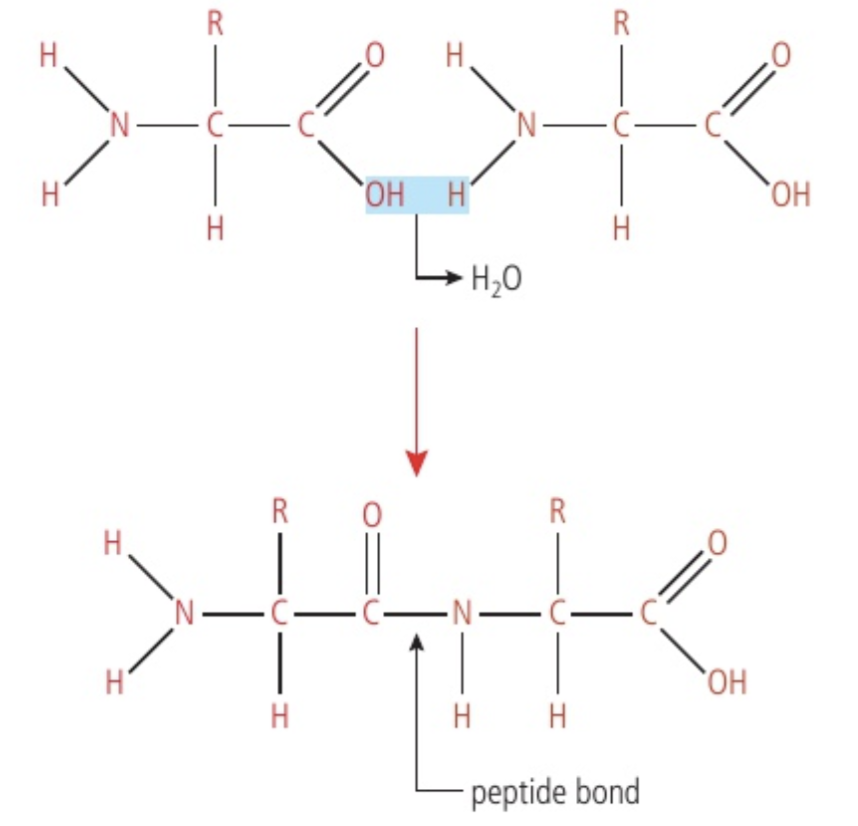
Digesting polymers into monomers
Food is digested into the alimentary canal, where the digestive enzymes that accomplish this are hydrolysing enzymes. Each individual reaction is called a hydrolysis reaction and requires a water molecule as a reactant. In a hydrolysis reaction, a water molecules always ‘split’ as part of the reaction.
What is the metabolism mainly composed of?
hydrolysis and condensation reactions, with enzymes to help with these reactions.
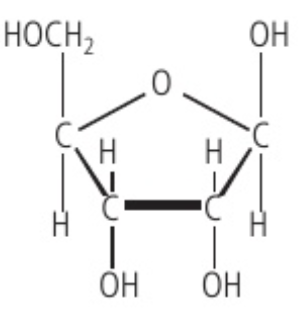
pentose monosaccharides
Ribose is an example of this structure, where it’s carbon backbone is composed of 5 carbons.
Chemical formula: C5H10O5
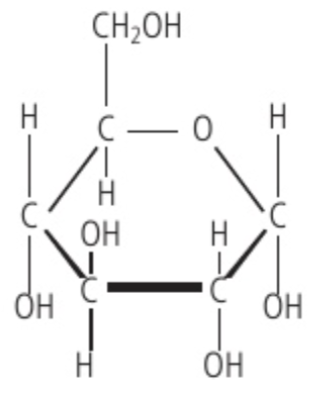
hexose monosaccharide
Glucose is an example of this structure, where it’s carbon backbone is composed of 6 carbons.
Chemical formula: C6H12O6
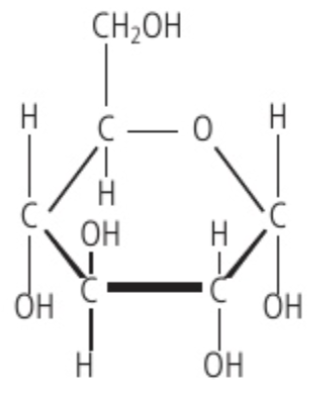
Properties of glucose
produced by photosynthesis & used in respiration
can be used to make polysaccharides (for structural & energy functions)
Due to glucose having 5 alcohol (-OH) functional groups, where OH has a polar bond, it makes glucose a polar molecule.
This polar covalent nature then leads it to have the following properties:
Easily soluble in water
Easily transportable in cells (due to solubility)
Yields a great deal of chemical energy
Has a lot of molecular stability
What is starch composed of?
polysaccharide comprising of hundreds of glucose monomers
how does starch stay compact?
Using two types of bonds between glucose molecules:
Alpha 1-4 linkage
Alpha 1-6 linkage
where the numbers refer to the carbon number of the two glucose molecules that are bonded together.
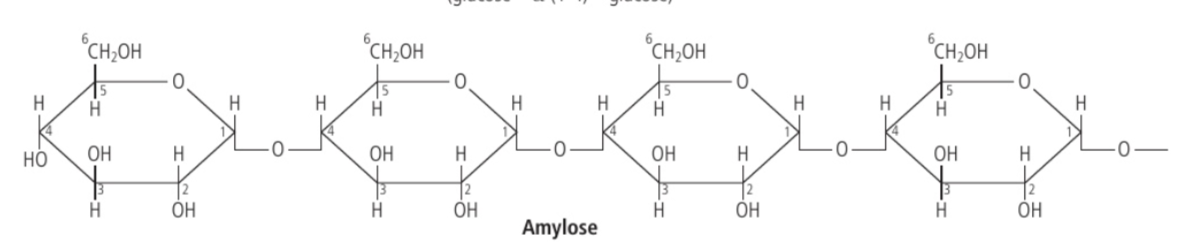
Amylose
a type of starch where the carbon #1 is bonded to the carbon #4 of adjoining glucose.
When hundreds of glucose molecules are bonded by only 1-4 linkages, the resulting structure will be linear but in a helix shape.
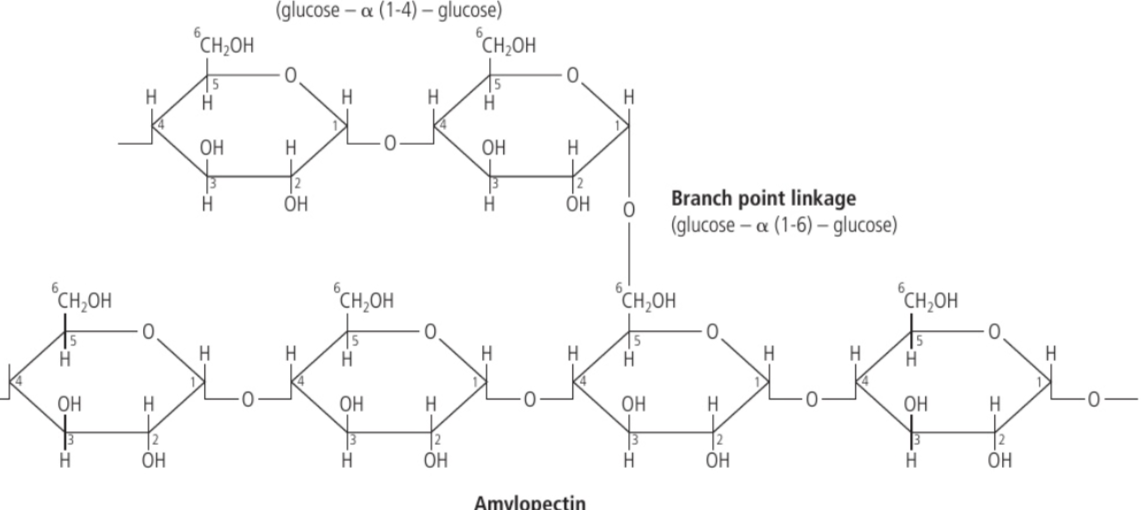
Amylopectin
a type of starch where the carbon #1 is bonded to the carbon #6 of adjoining glucose.
polysaccharides
major classes of biomolecules. They are long chains of carbohydrate molecules, composed of several smaller monosaccharides
monosaccharides
a carbohydrate consisting of one sugar unit. Common examples of simple sugars or monosaccharides are glucose and fructose.
What are the two polysaccharides of starch?
Amylopectin and Amylose
Glycogen
polysaccharide made of glucose monomers, with similar bonding patterns as in 1-6 amylopectin linkages.
Many animals (including humans) store extra glucose as glycogen, where the reserves are kept in our liver & muscle tissue.
Advantage for organisms storing glucose as a polysaccharide
the macromolecules are not readily soluble in cytoplasm and other fluids. This means that they do not affect the osmotic balance in living tissues, whereas individual (very soluble) glucose molecules would.
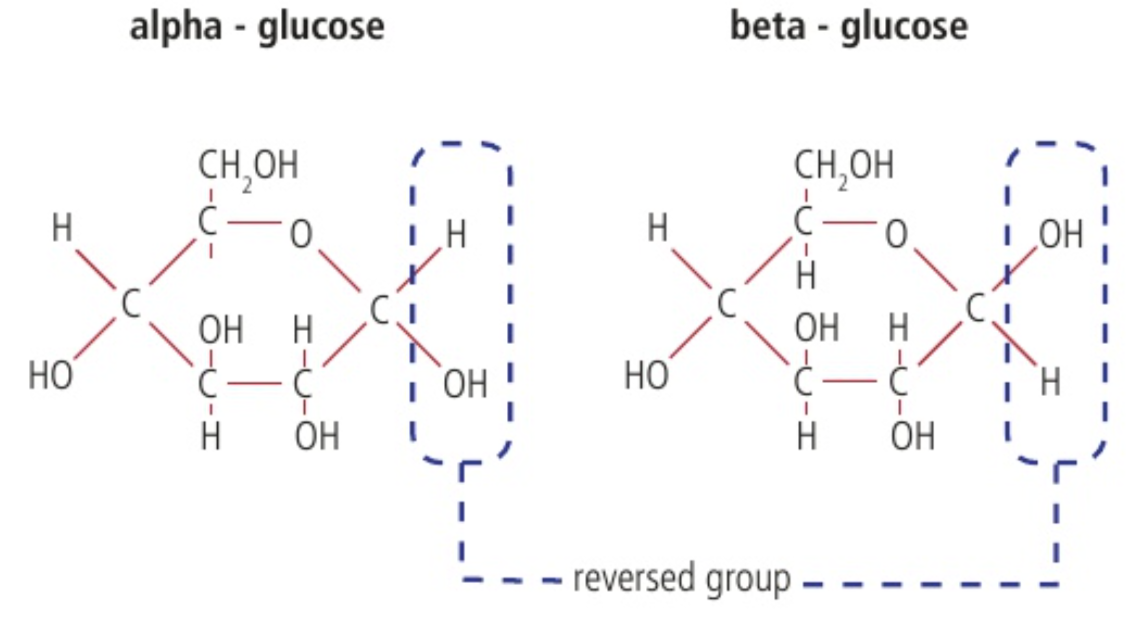
two forms of glucose (alpha and beta) and reversal of atoms
the reversal of atoms of the right side of each molecule is significant for the types of polymers that they form.
Starch and glycogen both use the alpha glucose
Cellulose use the beta glucose
Cellulose
primary component of cell walls of plants, estimated to be the most abundant of all organic molecules on earth.
its function is to act as a structural molecule in nature, like in plant cell walls. It is also insoluble in water and strong, where its fibres allow water and other substances to pass freely in and out of plant cells.
Conjugated carbon molecules
sometimes, some biochemically important molecules bond together to accomplish a certain function.
For example:
Lipoprotein: Lipid + Protein
Glycolipid: Carbohydrate + Lipid
Glycoprotein: Carbohydrate + Protein
Glycoproteins and cell-cell recognition
various kinds of proteins are associated with cell membranes, where these membrane proteins may or may not be conjugated.
These proteins carry out the following functions:
cell to cell chemical communication
transport of molecules in & out of a cell
cell to cell adhesion
recognition of body cells vs non-body cells for immune system functions
Glycoproteins and ABO blood types
the glycoproteins on the surface of red blood cell membranes can determine an individuals ABO blood type.
Red blood cells can have 2 types of glycoproteins on their cell membrane, where the A & B proteins are called antigens, and their presence trigger the immune system.
AB are universal recipients for blood, whereas O are universal donors of blood.
Blood type | Antigens present | What will immune system get triggered by? |
|---|---|---|
AB | Both antigens present | Neither antigen |
A | A antigens present | B antigen |
B | B antigens present | A antigen |
O | Neither antigens present | Both antigens |
Lipid solubility & organisms solution
Lipids, consisting of oils, fats, waxes and steroids, contain many areas of hydrocarbon (hydrogen and carbon), where they form a non-polar covalent bond. This means that they are hydrophobic, where they do not dissolve well in water.
In order to dissolve lipids, organisms will conjugate lipids with other molecules (such as glycolipids and lipoproteins)
triglycerides
Type of lipid (fat) found in your blood.
Contains:
1 glycerol + 3 fatty acids → 1 triglyceride + 3 water molecules
forms a triglyceride lipid through condensation reaction (as shown by 3 water molecules produced)
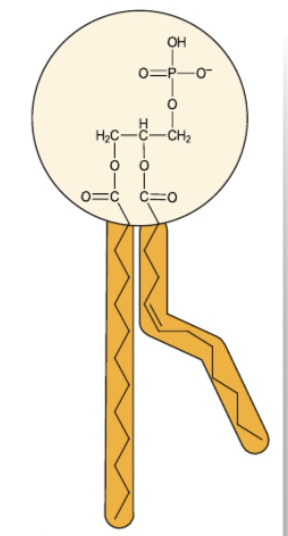
phospholipids
Type of lipid found in cell membrane
Contains:
1 glycerol + 2 fatty acids + 1 inorganic phosphate → 1 phospholipid + 3 water molecules
forms a phospholipid lipid through condensation reaction (as shown by 3 water molecules produced)
Glycerol
3 carbon molecule where each carbon bonds to one hydroxyl group (initially)
Fatty acids
depend on:
# of carbons
possible presence of double bonds between carbons
each of these molecules contains a terminal carboxyl group involved in the condensation reaction.
Properties of fatty acids (three groups)
Saturated Fatty Acids | Monounsaturated fatty acids | Polyunsaturated fatty acids |
|---|---|---|
single carbon bond | one double carbon bond | many double carbon bond |
high melting point & solid at room temp | lower melting point & oil at room temp | low melting point & oil at room temp |
found in animal meat & butter | some animals & plants store energy in this form | many plants store energy in this form |
Adipose tissue
composed of cells that store fat in the form of triglycerides.
its quantity is determined by the calories consumed by an organism, where there will be an increase with more calories consumed than burned.
Thick layers of adipose tissue is common for animals that live in cold climates, where these animals are called endotherms.
endotherms
those that maintain a constant body temperature independent of the environment.
Found in animals like whales, seals and walruses, there is a thick adipose tissue called blubber and is found between their skin and muscles, helping to trap heat generated by their internal metabolism.
adipocyte
fat storage cell
triglycerides function
can supply energy when there is not enough food, where the stored triglycerides will undergo hydrolysis reactions where the products (glycerol and fatty acids) are used for energy in cell respiration.
It is useful for long term energy storage, as they are insoluble in body fluids and thus do not move from their adipose storage sites.
phospholipid bilayer
phospholipids have a polar end (end with the phosphate group) and a non-polar end (end with the hydrocarbon tail)
This causes it to have both hydrophobic & hydrophilic regions, being known as an amphipathic molecule.
In aqueous solutions, such structures will arrange themselves in a bilayer (two sets), where the hydrophobic region will remain internal, while the hydrophilic region will remain external. This then forms a plasma membrane, found in many organelles.
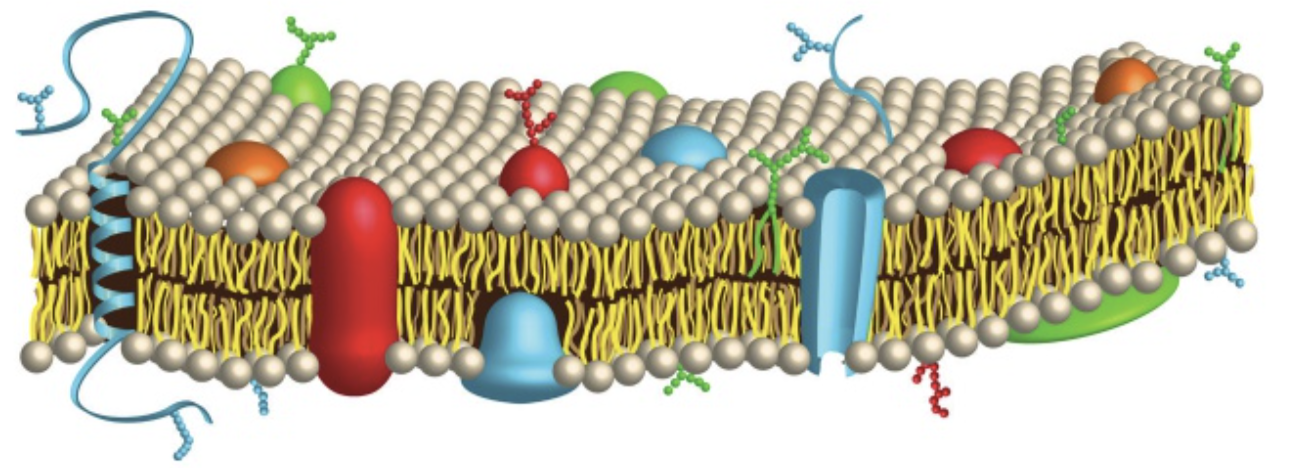
amphipathic molecules
molecules containing both polar and non-polar (apolar) portions in their structure.
Hormones
Hormones are chemical messenger molecules that are produced by many glands in the body.
Once they are produced, they have access to all body tissue, where the tissue can then respond to that hormone.
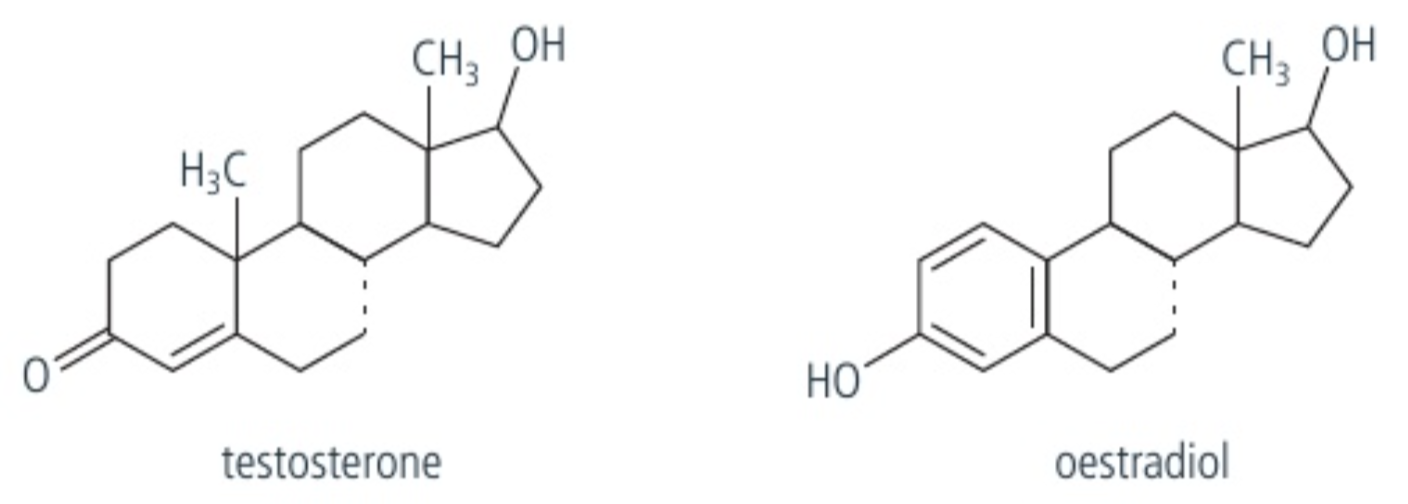
Steroid hormones
hormone that is made from the lipid cholesterol, which is primarily a hydrocarbon molecule.
Two types of steroids:
Oestradiol
Testosterone
these two steroids are produced by a gonadal tissue, and are involved in the development of primary and secondary sex characteristics in puberty. As they are lipid molecules, they can easily pass through the plasma & nuclear membrane and direct the process of transcription, leading to production of mRNA molecules.