Chemistry 101 Exam 2 (Travis Varner)
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
polar covalent bond
when 2 non metal atoms share electronegativity
ionic bonds
difference in electronegativity is large, occurs between a metal and nonmetal
non polar covalent bond
difference in electronegativity is 0 occurs between two of the same non metals or between carbon and hydrogen
1
mono
2
di
3
tri
4
tetra
5
penta
6
Hexa
7
hepta
8
octa
9
nona
10
deca
formal charge
# of valence electrons - # dots - # lines
hydrogen bonding
one molecule with a H-N, H-O, or H-F bond and a second molecule with a N,O, or F atom
calcium nitrate
Ca(NO3)2
chromium(II) oxide
CrO
aluminum carbonate
Al2(CO3)3
iron(II) chloride
FeCl2
K2O
Potassium oxide
CoCl3
Cobalt(III) chloride
Fe(NO3)3
Iron(III) nitrate
NH4ClO
Ammonium Perchlorate
Covalent, Ionic, or both
HC2H3O2
covalent
Covalent, Ionic, or both
CaSO4
both
Covalent, Ionic, or both
MgCl2
ionic
polar, nonpolar, or ionic
Si-Si
nonpolar
polar, nonpolar, or ionic
S-C
polar
polar, nonpolar, or ionic
Rb-O
ionic
How many moles of elemental oxygen gas (O2) must react with elemental phosphorus (P4) to produce 62.3 moles of diphosphorus pentoxide?
How many moles of elemental oxygen gas are required to produce 56.9 moles of nitrogen monoxide?
How many moles of hydrogen atoms are present in 13.9 moles of ammonia (NH3)?
155.8
28.5
41.7
4 grams of a gas at 200 K and 8 atmospheres occupies a volume of 20 liters. Use relationships from Avogadro's law, Boyle's law, Charles's law, and the ideal gas law to solve this problem.
160
To what temperature must a 2.24 L container filled with 9.39 g of Kr be heated in order to obtain a pressure of 1.22 atm?
297
What mass of helium is required to fill a 1.41-L balloon at 47.49°C and 5.00 atm?
1.07
What are the concentration and pressure of Ar in a 26.0-L flask at 480. K that contains 21.0 g of Ar?
concentration - 0.0202
pressure - 0.796
Indicate which molecule (a or b) in each pair has the higher boiling point. Also indicate which force, hydrogen bonding (H), dipolar (P), or dispersion (D) is most responsible for the difference. For part 2 of this problem, you can see the Lewis structures for those molecules listed at the bottom of this problem.
NH3 or PH3
NH3
hydrogen bonding
Indicate which molecule (a or b) in each pair has the higher boiling point. Also indicate which force, hydrogen bonding (H), dipolar (P), or dispersion (D) is most responsible for the difference. For part 2 of this problem, you can see the Lewis structures for those molecules listed at the bottom of this problem.
C4H8 or C10H20
C10H20
dispersion
Indicate which molecule (a or b) in each pair has the higher boiling point. Also indicate which force, hydrogen bonding (H), dipolar (P), or dispersion (D) is most responsible for the difference. For part 2 of this problem, you can see the Lewis structures for those molecules listed at the bottom of this problem.
HCl or F2
HCl
dipole-dipole
What mass of KCl is required to make 47.0 mL of a 0.260 M KCl solution?
How many moles of potassium ions are present in the solution?
0.894
0.012
a) How many moles of Al2(SO4)3 are required to make 59 mL of a 0.060 M Al2(SO4)3 solution? Remember that 1000 mL = 1 L.
What mass of Al2(SO4)3 is required to make 59 mL of a 0.060 M Al2(SO4)3 solution?
How many moles of sulfate ions are present in the solution?
0.00354
1.21
0.01062
What is the sodium ion concentration in a solution prepared by mixing 0.354 mol Na2SO4 in enough water to make 1.40 L of solution?
0.506
Write the chemical formula of the precipitate (not the net ionic equation) that would form when the following solutions are mixed, or write "NONE" if no precipitate would be produced.
sodium chromate + nickel(II) iodide
NiCrO4
Write the chemical formula of the precipitate (not the net ionic equation) that would form when the following solutions are mixed, or write "NONE" if no precipitate would be produced.
sodium sulfide + iron(III) chloride
Fe2S3
Write the net ionic equation (not just the chemical formula of the precipitate) for the reactions that occur when solutions of the following salts are mixed. (Enter "NONE" if no reaction occurs. Use the lowest possible whole number coefficients. Omit states-of-matter from your answer.)
K3PO4 + Cu(ClO4)2
3Cu2+ + 2PO
3-4 →Cu3(PO4)2
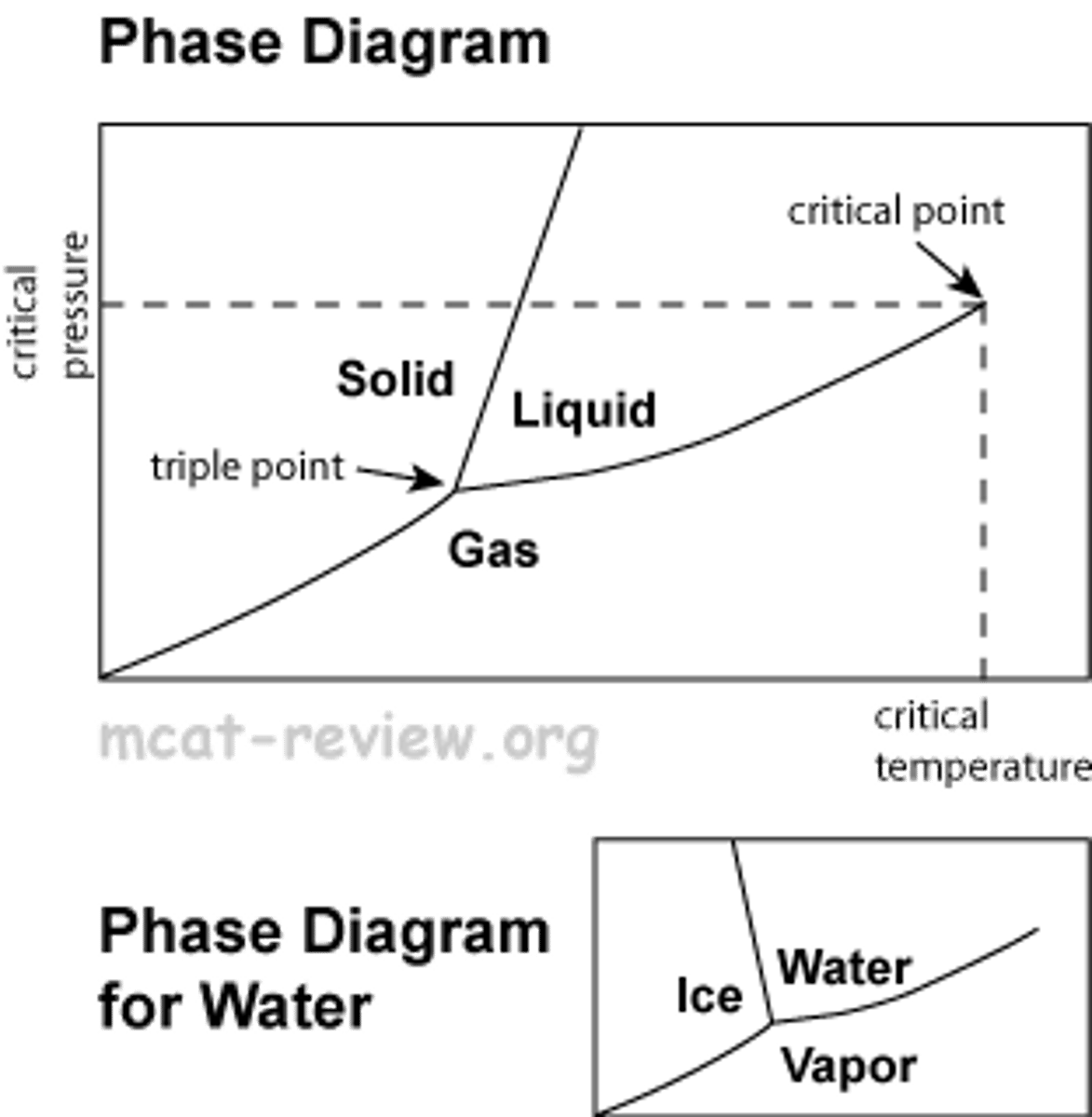
phase diagram
You need to prepare 4.0 L of a 2.0M N(CH3 )3 solution in water.
How many L of N(CH3 )3 are needed?
How many L of water are needed?
Density of N(CH3 )3 670 grams/L
Molar mass of N(CH3 )3 59.11 grams/mole
Density of water 1000 grams/L
Molar mass of water 18.02 grams/mole
0.71
3.29
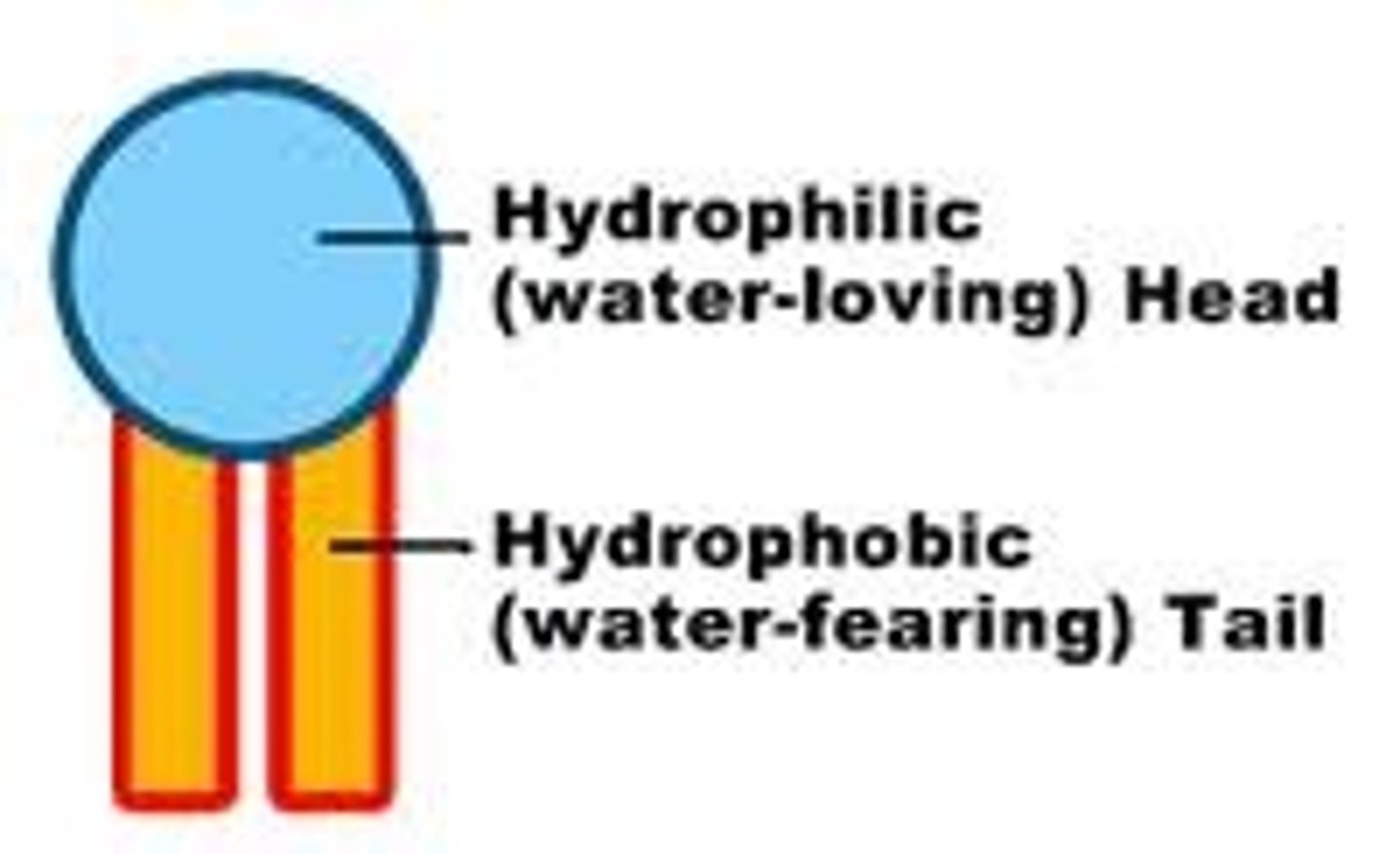
hydrophobic tail
Water fearing
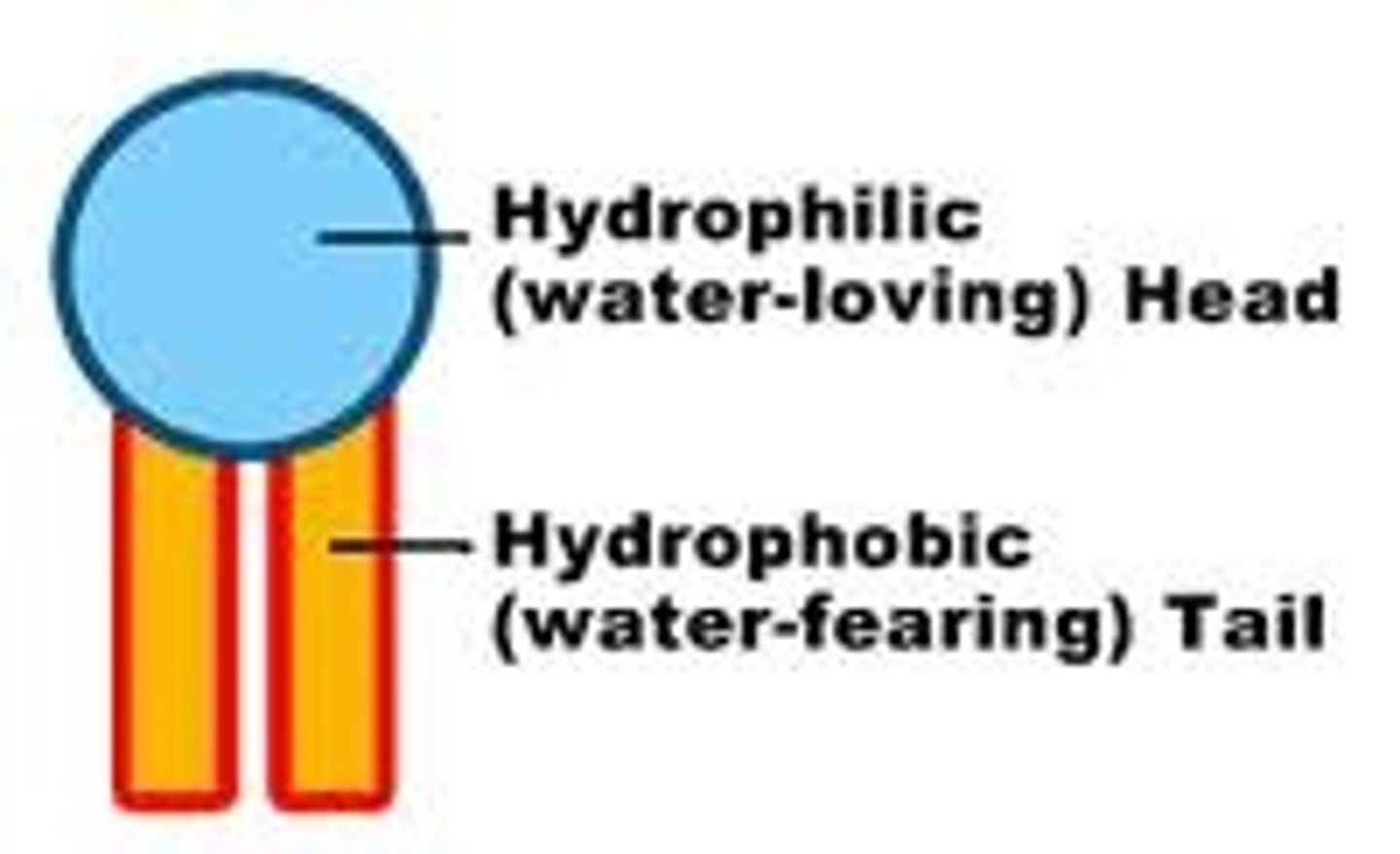
hydrophilic head
water loving