Chem 2C Midterm 1
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
Cathode
Reduction
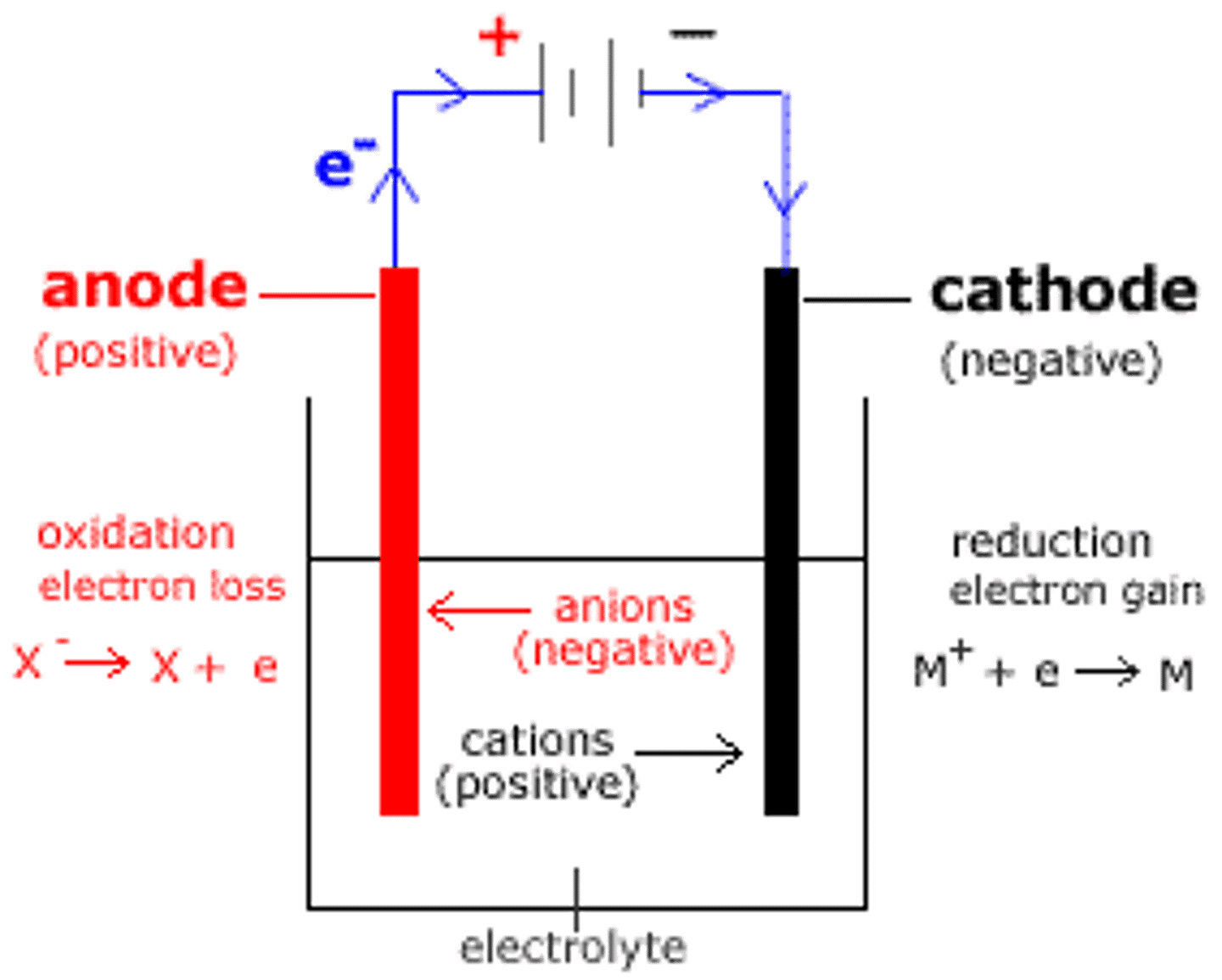
Anode
Oxidation
Primary Cell Batteries
Cannot be recharged (dry cell batteries, silver-zinc batteries)
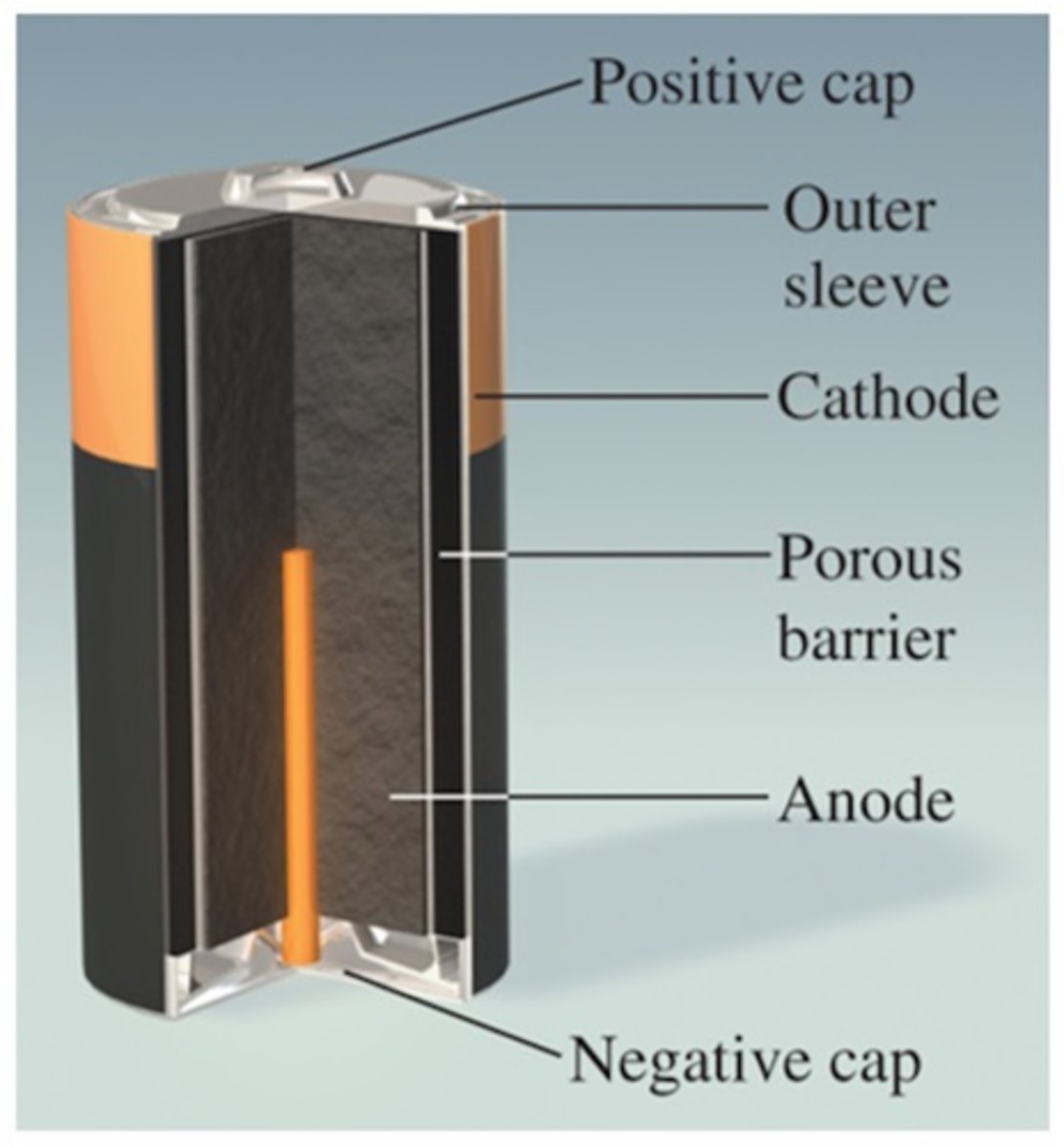
Dry Cell Batteries
Acidic or alkaline, anode = zinc and cathode = manganese oxide. Hold charge a long time, and good for emergency use

Silver-Zinc/Button Batteries
Small with a high-storage capacity, very toxic if swallowed, anode = zinc and cathode = silver
Secondary Cell Batteries
Can be recharged, cell reaction reversed with electricity
Lead-Acid/Storage Batteries
Concentration cell with HSO4 and lead, used for car batteries, can last for years, can explode if jumped incorrectly, needs to be topped off with water as HSO4 concentration changes over time, can't start at low temps due to contraction

NiCad Batteries
Home-use rechargable batteries and have memory, alkaline, anode = cadmium and cathode = nickel

Lithium Battery (Li-Ion Cell)
Reliable with long lifetime, twice the capacity of NiCad batteries, avoid heat, used in pacemakers and laptops and more, have potential for deep discharge but varies. Cathode and anode are different kinds of lithium ions/compounds

Flow/Fuel Cell Batteries
Converts chemical to electrical energy, fuel and oxygen, used by power-plants and NASA, cell burns cleanly with only water as a product, spontaneous, platinum is used as a catalyst, not really a battery

Air Batteries
Water and aluminum used as reactants, cathode is oxygen, aluminum from reaction recycled and reused, must be reset every 250 miles (add 6 gallon of water, remove aluminum hydroxide)

Corrosion
Oxidation of metal, for example rust where cathode = oxygen and water and anode = iron

Plating
putting a sacrificial anode onto another metal to prevent corrosion

Alloy
Mixture of metals, homogeneous or not

Cathode Protection
technique for controlling corrosion of a metal

Sacrificial Anode
Oxidizes first before metal it's protecting

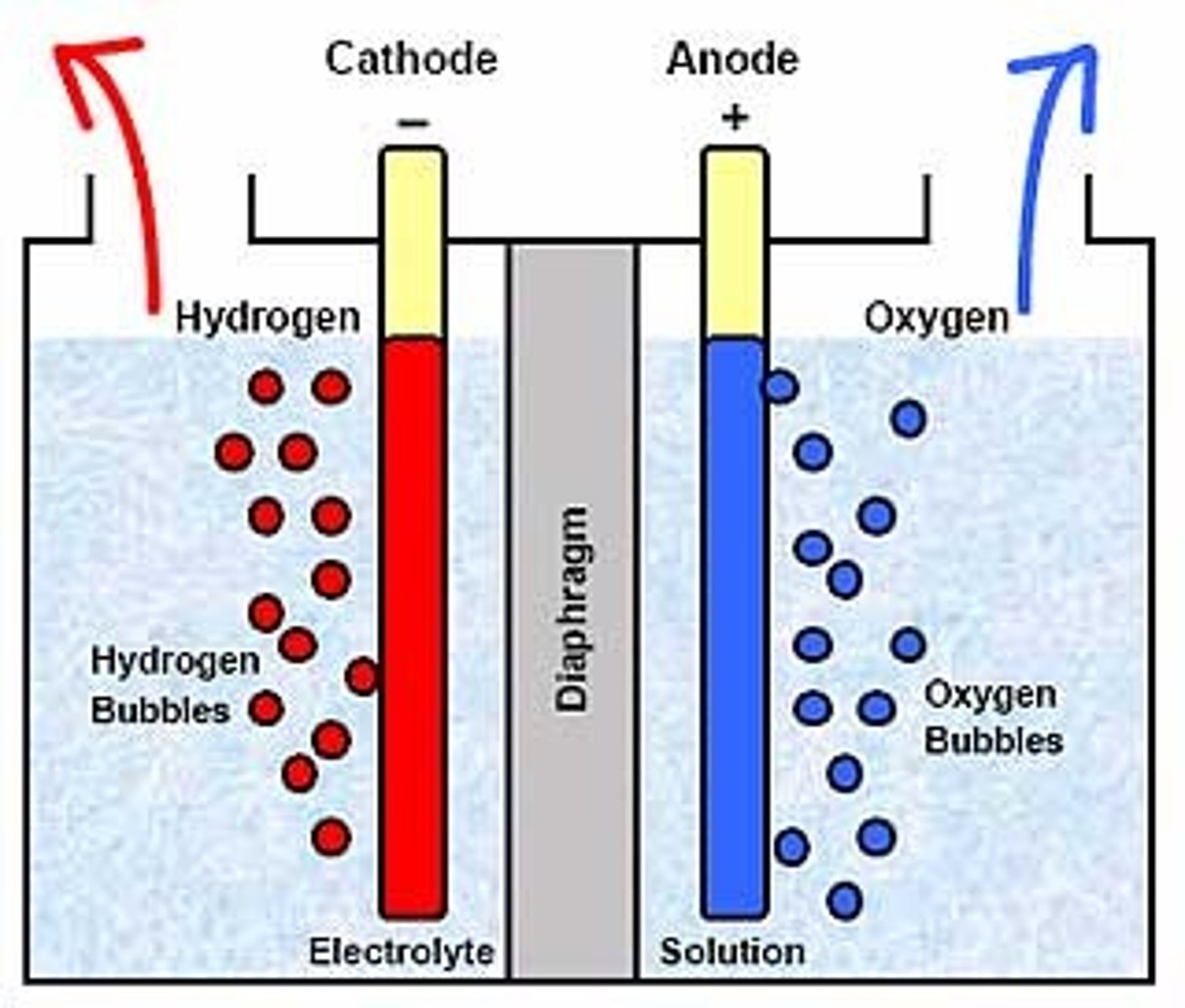
Electrolysis
Forcing a non-spontaneous reaction to occur via electricity
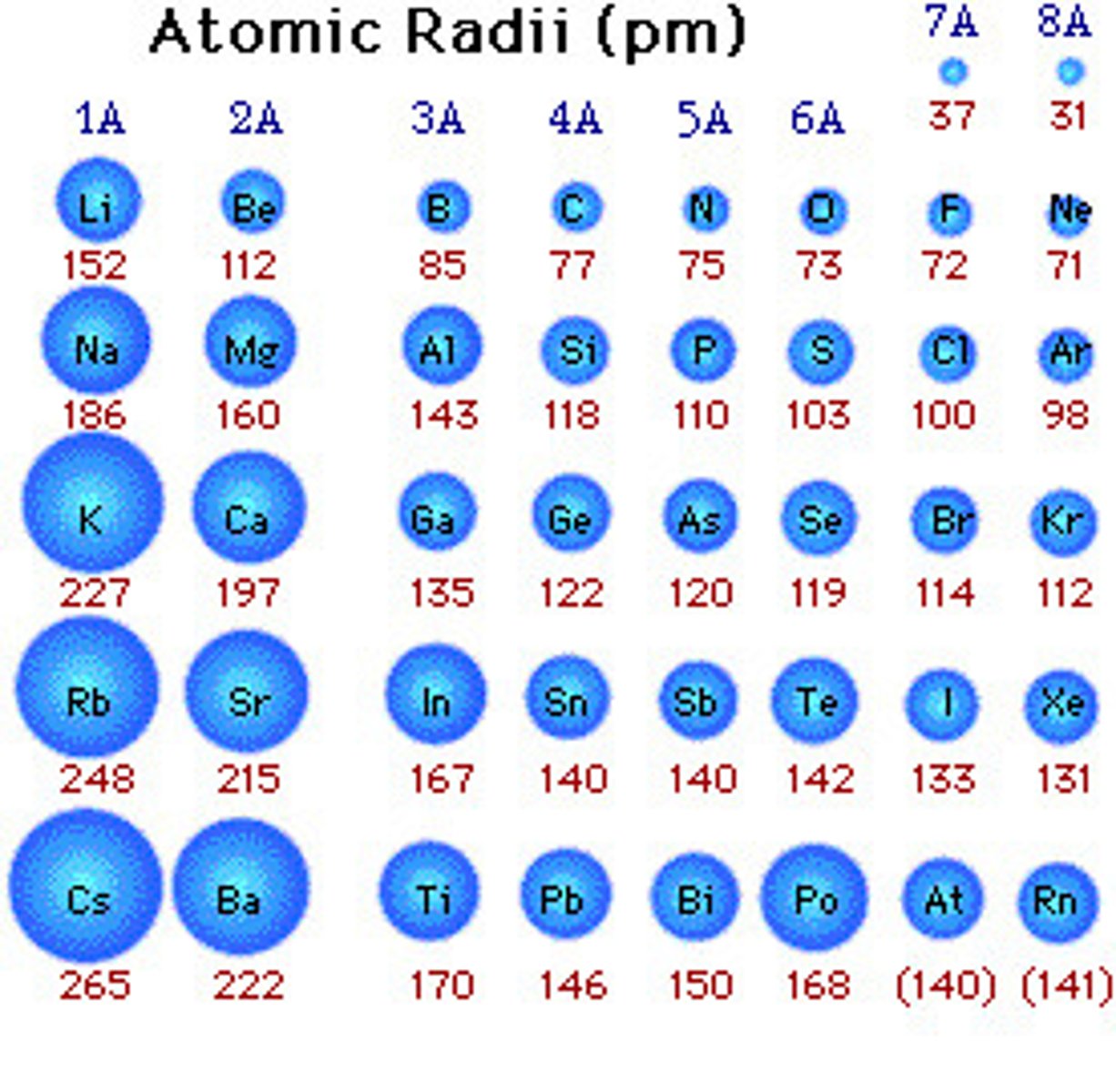
Radii
decrease and then increase across row, first row is small and second and third rows are approximately the same because of lanthanide contraction
Density
increases then decreases across the row, and third row has significantly higher densities than other rows because of lanthanide contraction
Transition Metal Character
ionic and covalent
Color
Partially filled d shells give color, and fully filled or unfilled ones are colorless or white
Magnetism
Paramagnetic or ferromagnetic

Catalysts
TMs are good catalysts because can accept a lot of ligands easily and varying ox. states and CNs

Metallurgy
Process of making pure metal from ore

Scandium
Scandium family, +3, d0, colorless, dimagnetic, has diagonal relationship to aluminum so alloys are made between them

Titanium
Titanium family, +4, diagonal relationship to carbon and silicon, low density and high strength, white pigment in paper, used for artificial joints

Vanadium
Vanadium family, +2 and +5, V2O5 (vanadium oxide) is a common catalyst for production of sulfuric acid, hard

Chromium
Chromium family, +2 and +3 and +6, good protective material for plating, terracotta soldier weapons plated with chromium, red color in rubies

Tungsten
Chromium family, only third row element found in biomolecules, heavy and strong and hard, drill bits, light-bulb filaments

Manganese
Manganese family, +2 to +7, MnO2 and MnO4-, dry cell batteries, good catalyst

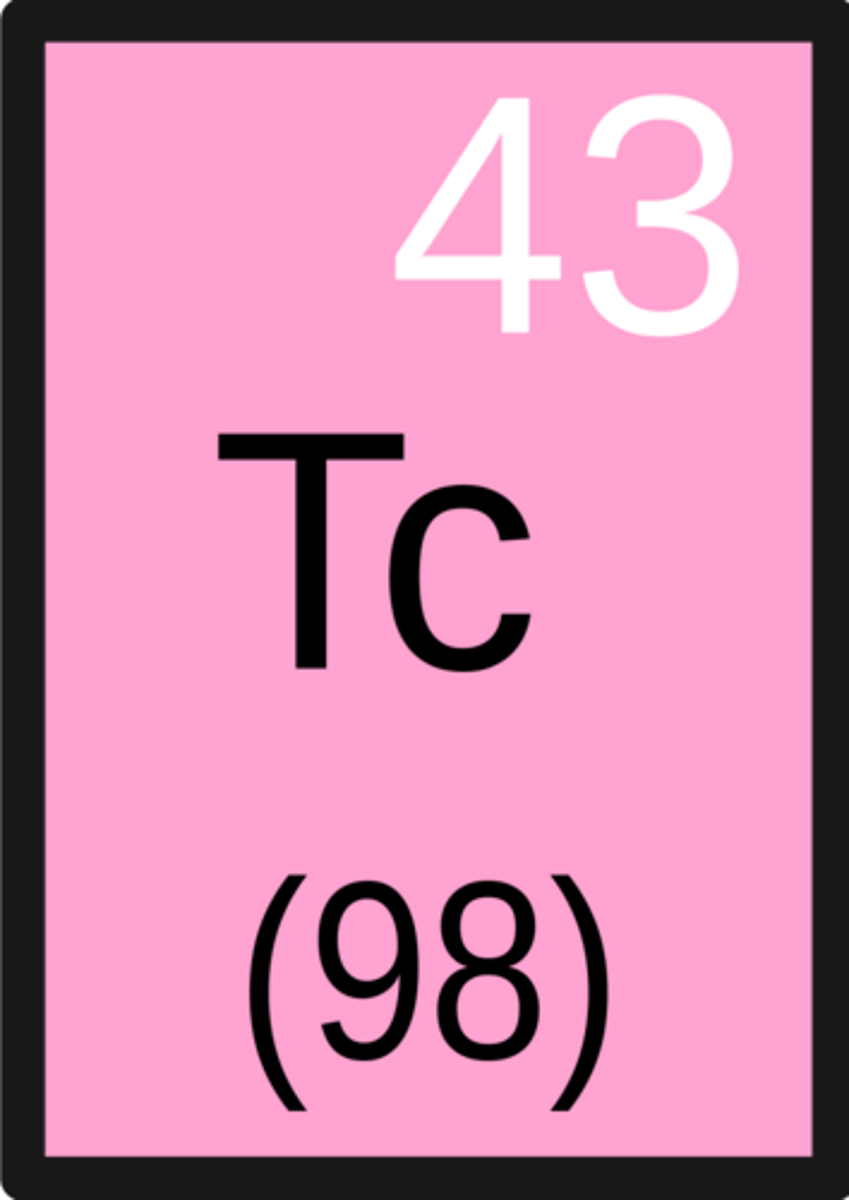
Technetium
Manganese family, smallest fully radioactive compound, tracer for mammograms
Iron
Iron family, +2 and +3, most abundant on Earth (4.7%), alloys, steel structures, metal-carbonyl bonds

Cobalt
Cobalt family, +2 and +3, blue color but also range of colors, batteries, vitamin B-12, metal-carbonyl bonds

Nickel
Nickel family, resists corrosion, +2, coining metal, NiCad batteries, good catalyst, double-magic metal-carbonyl bonds

Platinum
Nickel family,+2 and +4, least reactive metal, good catalyst, does not oxidize readily

Copper
Copper family (coinage metals), +1 and +2, alloys are bronze (tin + copper) and brass (zinc + copper), very conductive (copper wires), coinage metal

Silver
Copper family, +1, alloys (mercury + silver tooth fillings), coinage metal, jewelry

Gold
Copper family, +1 and +3, key to monetary system, does not oxidize readily

Zinc
Zinc family, sacrificial anode, colorless (d10), batteries

Mercury
Zinc family, +1 and +2, quicksilver, Hg because hydrargyrum, toxic!

Lanthanides
Zinc family (?), +3 (sometimes +2 and +4), comes in ores with multiple lanthanides in them
Actinides
Zinc family (?), paramagnetic and radioactive, toxic, similar to lanthanides, nuclear weapons
CO
carbonyl
NO
Nitrosyl
CH3NH2
methylamine
C5H5N
Pyridine
O (2-)
oxo
OH-
Hydroxo
CN-
Cyano
SO4 (2-)
sulfato (sulfate)
S2O3 (2-)
Thiosulfato
NO2 (-)
Nitrito-N-
ONO (-)
Nitrito-O-
SCN (-)
Thiocyanato-S-
NCS (-)
Thiocyanato-N-
En
ethylenediamine (bidentate)
ox (2-) or C2O4 (2-)
Oxalato (bidentate)
EDTA (4-)
ethylenediaminetetraacetato (Hexadentate)
Iron
ferrate
Copper
cuprate
tin
stannate
silver
argentate
lead
plumbate
gold
aurate
Structural isomers
Coordination, linkage, ionization
Linkage Isomers
Ligand connecting with different atoms (ex: SCN vs CNS)
Ionization isomers
Ligand and counter ion swap
Coordination isomers
Two transition metals in same complex swap partners/ligands
cis
different axes
trans
same axes, never chiral
fac
same face
mer
same plane/neighboring, never chiral