Environmental Biotech post-mids
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
Components of domestic wastewater
Bathrooms and kitchens supply fats, oils, grease (FOG) which float and clog pipes; grease traps are used in pre-treatment.
Laundry wastewater contains surfactants, phosphates, microplastics, and bleaching chemicals.
Households now contribute pharmaceutical residues and personal care product chemicals — modern tertiary treatment (activated carbon, membranes) is needed for these.
Seasonal variation: more water usage in summer; peak loads occur in mornings and evenings.
Grey water vs Blackwater
Greywater has lower pathogen load, so it can be reused for toilet flushing, irrigation, and groundwater recharge after light treatment.
Blackwater has higher ammonia and fecal coliform counts, requiring full biological treatment + disinfection.
In some countries, separate plumbing systems recover greywater to reduce treatment plant load by up to 40%.
Greywater reuse reduces stress on water-scarce regions (Middle East, Pakistan rural setups).
Importance of wastewater: environmental impact
Prevents oxygen depletion in rivers/lakes, avoiding aquatic life death due to low dissolved oxygen.
Stops eutrophication by removing nitrogen and phosphorus, which trigger algal blooms and dead zones.
Reduces release of toxic household chemicals, pharmaceuticals, microplastics, and personal-care products, which harm fish and disrupt endocrine systems.
Importance of wastewater treatment
Removes pathogens (bacteria, viruses, protozoa, helminths), preventing cholera, dysentery, typhoid, hepatitis A/E.
Keeps contaminants out of drinking water sources and agricultural irrigation canals.
Controls mosquito breeding: untreated sewage creates stagnant anaerobic pools that support vectors of malaria and dengue.
Name the stages of wastewater treatment
Stages of Treatment
- Primary Treatment: Removes large solids and debris through physical methods.
- Secondary Treatment: Uses biological processes to break down organic pollutants.
- Tertiary Treatment: Employs advanced methods for removing residual pollutants and nutrients.
- Sludge Treatment: Focuses on processing and disposing of solid waste generated during treatment.
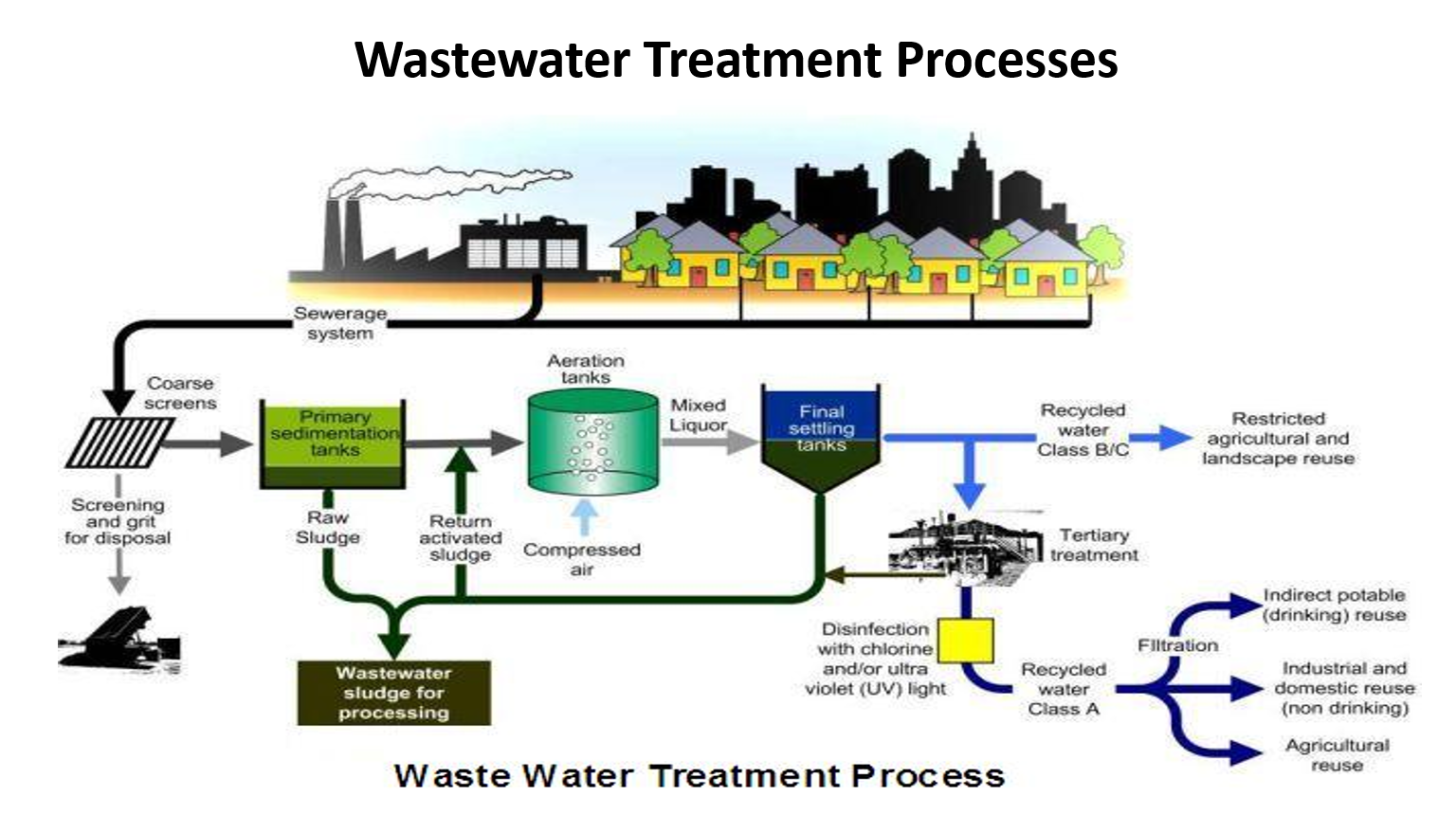
Draw this diagram
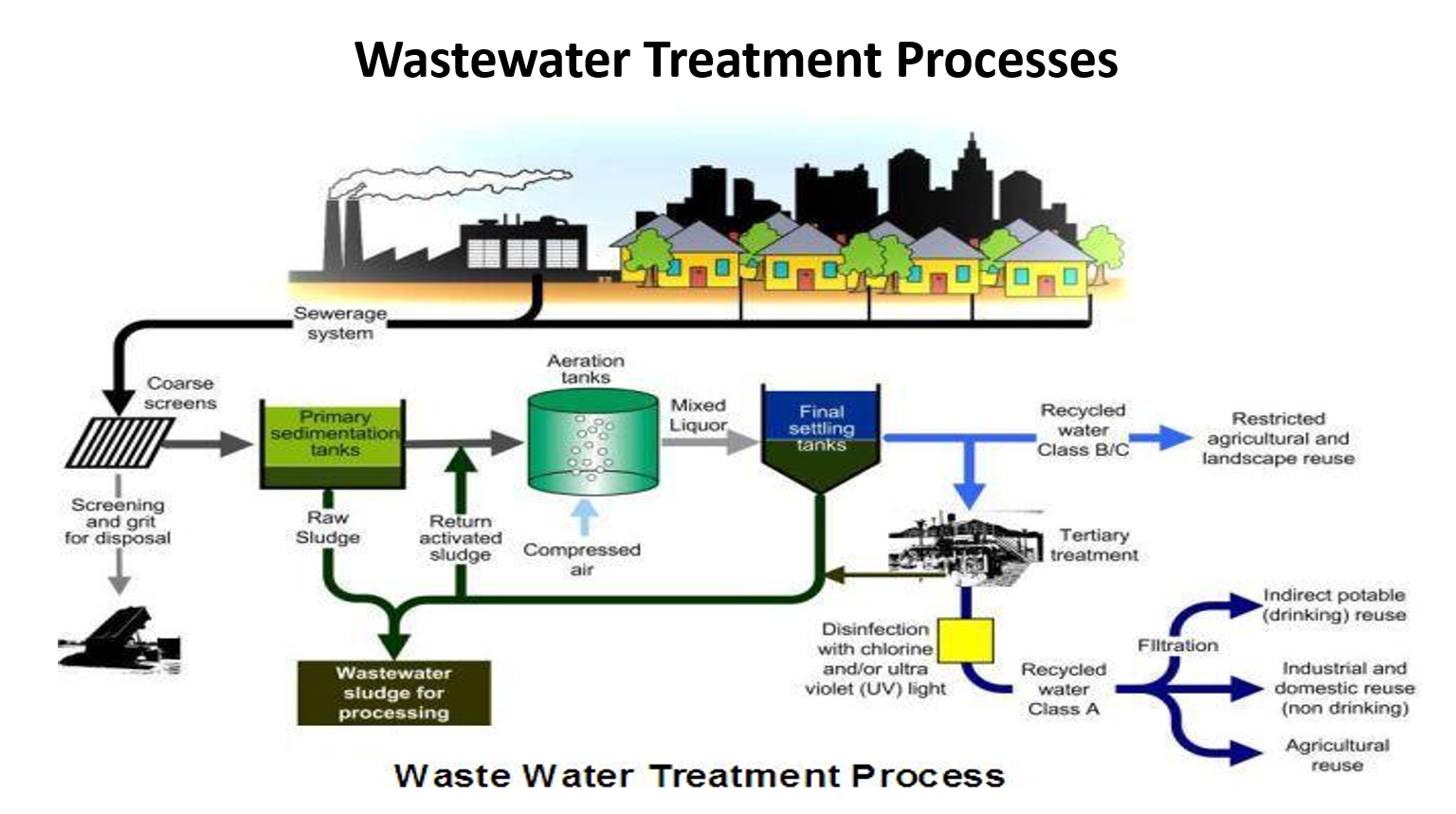
Explain until primary sedimentation
✅ 1. Wastewater enters from houses
All sewage from kitchens, bathrooms, toilets flows into a sewerage system and reaches the treatment plant.
✅ 2. Coarse Screening
A metal bar screen removes large objects: plastics, cloth, rags, sticks, stones.
A grit chamber removes sand and gravel.
These solids are sent for landfill disposal.
Why this matters:
Big items can damage pumps, block pipes, and disturb downstream treatment.
✅ 3. Primary Sedimentation Tanks
Water flows into a large settling tank.
Gravity settles heavy solids → this forms raw sludge at the bottom.
Grease and oils float and are skimmed off.
Outcome:
Removes 50–60% of suspended solids and about 25–35% of BOD.
Raw sludge is sent for sludge treatment (shown at bottom).
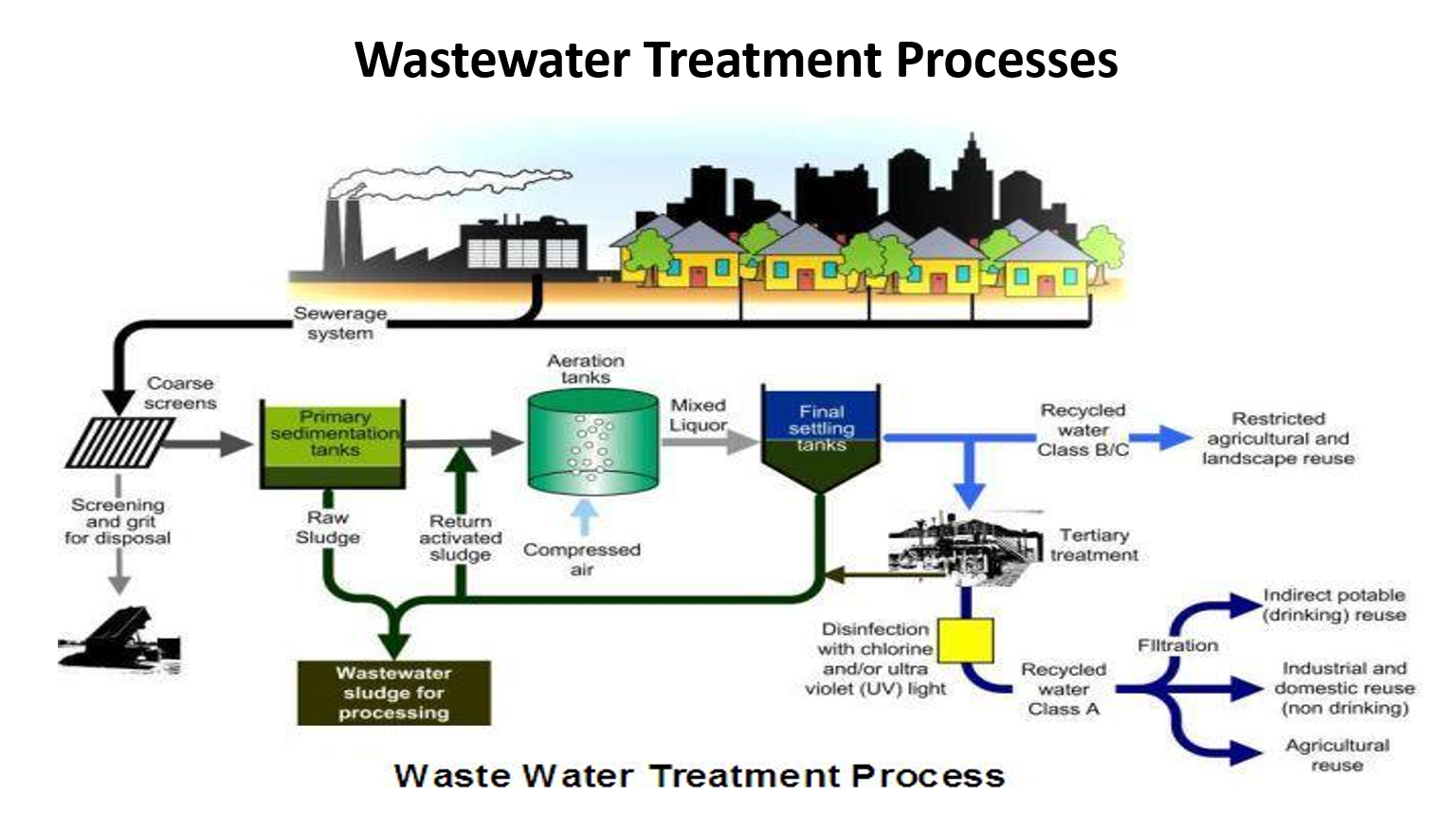
explain until final settling tanks
✅ 4. Biological Treatment – Aeration Tanks
This is the activated sludge process.
Compressed air (oxygen) is bubbled in.
Microorganisms (bacteria) eat organic pollutants and multiply, forming mixed liquor (water + biomass).
🧪 Reaction:
Organic matter → CO₂ + H₂O + new microbial biomass
✅ 5. Final Settling Tanks (Secondary Clarifier)
Mixed liquor flows here.
Biomass/flocs settle at bottom.
Clear water rises and exits.
Two flows happen:
✅ Clear water → goes to next stage
✅ Settled sludge → a part is returned to aeration tank as Return Activated Sludge (RAS)
✅ Excess sludge → sent for processing (WAS)
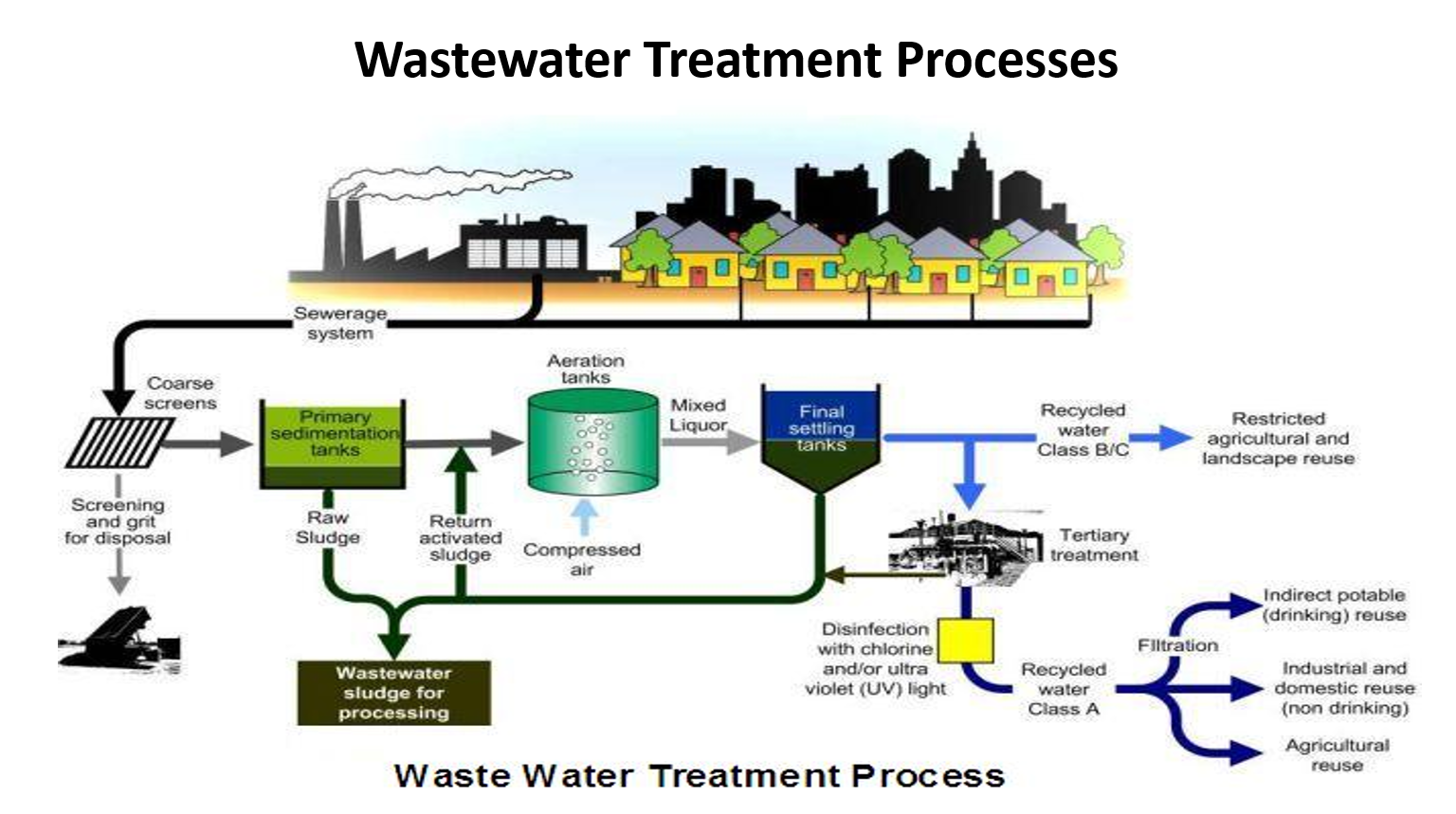
explain the last 3rd of the process
✅ 6. Disinfection (Tertiary Treatment)
Remaining pathogens are killed using chlorine or UV light.
Produces Recycled Water – Class A (high quality).
✅ 7. Filtration
Sand or membrane filters remove tiny particles and turbidity.
Activated carbon can remove taste/odor, pesticides, pharmaceuticals.
✅ 8. Different Types of Reuse
After tertiary treatment, water can be reclassified:
Class B/C Recycled Water
Lower treatment level
Used for restricted irrigation, parks, landscapes, non-food crops
Class A Recycled Water
Fully disinfected + filtered
Used for:
✅ Agricultural irrigation
✅ Industrial cooling
✅ Toilet flushing
✅ Some cities use it for indirect potable reuse (treated water goes to groundwater, later pumped again for drinking)
✅ 9. Sludge Handling
All sludge from primary and secondary treatment → goes to sludge processing
Often treated anaerobically → produces biogas for electricity and fertilizer
Explain primary treatment (screening)
The first step of wastewater treatment is coarse screening.
Metal bar screens (steel bars placed a few centimeters apart) are installed at the inlet of the treatment plant.As wastewater flows through, large solid objects like:
plastics and food wrappers
rags and clothing pieces
wood pieces, leaves, sticks
sanitary products, rubber, stones
get physically trapped on the screen surface.
These objects are removed mechanically or manually and sent to a landfill or incineration.
Purpose:
✅ Prevent clogging of pipes and pumps
✅ Protect delicate equipment (aerators, clarifiers) from damage
✅ Reduce maintenance costs
So, screening is a purely physical process with no biological or chemical treatment involved.
Explain primary treatment
After screening, wastewater still contains a lot of suspended solids, sand, silt, grease, and organic particles.
It flows into large sedimentation tanks (also called primary clarifiers).
The water is kept very still so particles can settle down by force of gravity.
Heavy solids sink to the bottom and collect as primary sludge.
Lighter particles, oils, and fats float on the surface and get skimmed off.
The clarified water (called primary effluent) flows to biological treatment.
✅ Primary sludge is thick, organic, and full of microorganisms.
It is pumped to sludge treatment units (anaerobic digesters, dewatering, drying beds).
Efficiency:
Removes ~50–60% of suspended solids
Reduces BOD by ~25–35%
Importance of primary treatment
Reduces the volume of solid waste entering biological treatment:
By removing large debris during screening and settling out heavier particles during sedimentation, the amount of solid material that reaches aeration tanks becomes much lower. This prevents overloading the biological system and keeps the treatment process stable.Prevents clogging of pumps and pipes:
Large items like plastics, cloth, and wood can jam mechanical parts and block flow channels. Screening protects pumps, aerators, mixers, and clarifiers from damage and interruption, allowing the plant to run smoothly without frequent shutdowns for repairs.Lowers the organic load for later stages:
Primary sedimentation removes a significant amount of organic matter (measured as BOD and suspended solids). With less organic pollution left, microorganisms in the biological stage can treat the wastewater more efficiently and achieve higher quality effluent with less energy and aeration demand.
Role of microbes in secondary treatment
In secondary treatment, aerobic bacteria and other microbes (protozoa, fungi) feed on organic pollutants present in wastewater.
They oxidize dissolved organic matter and convert it into:
✅ Biomass (new microbial cells)
✅ Carbon dioxide (CO₂)
✅ Water (H₂O)Because the microbes remove organic pollution, the Biochemical Oxygen Demand (BOD) of the wastewater decreases significantly.
Lower BOD means the treated water will not deplete oxygen in rivers or lakes and will not harm aquatic life.
Secondary treatment: activated sludge process
1. Activated Sludge Process
Wastewater enters aeration tanks, where compressed air or mechanical aerators are used to supply oxygen.
Aerobic bacteria form clumps called flocs that capture and digest organic matter.
The mixture of wastewater + microbes is called mixed liquor.
This then flows to a final settling tank where flocs settle to the bottom.
Settled biomass is handled in two ways:
✅ Some is returned to aeration tanks as Return Activated Sludge (RAS) to keep microbial population high.
✅ Extra sludge is removed as Waste Activated Sludge (WAS) for sludge treatment.This process removes 85–95% of BOD, providing high-quality effluent.
Secondary treatment: trickling filters
Wastewater is sprayed or distributed over a bed of rocks, gravel, or plastic media.
Microorganisms grow as a biofilm on the media surface.
As wastewater trickles over it, the microbes degrade organic pollutants.
Air circulates naturally, providing oxygen for aerobic respiration.
The treated water is collected at the bottom and sent to a settling tank.
Advantages:
Simple, low energy use, good for smaller cities or rural areas.
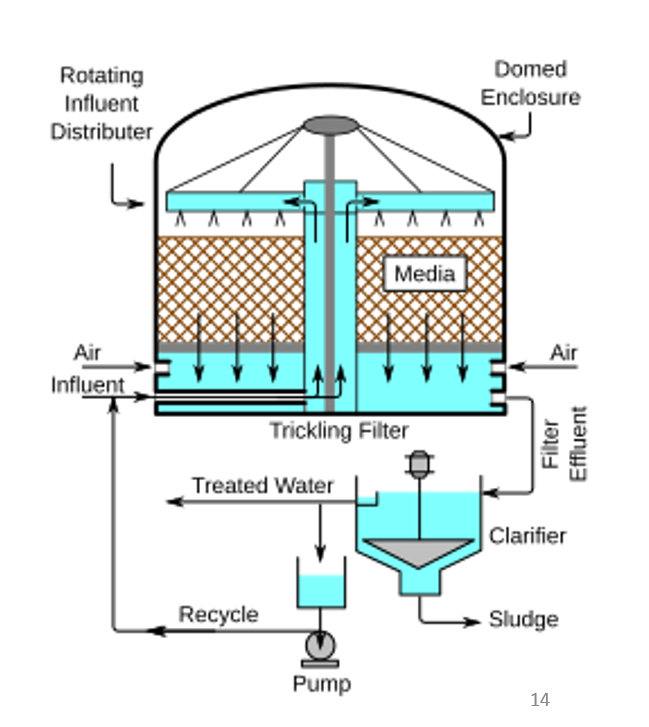
Trickling filters diagram
✅ 1. Wastewater enters at the top
The wastewater flows into a rotating influent distributor.
This rotating arm spreads wastewater evenly across the top of the filter.
Spreading is important so every part of the media gets equal contact.
✅ 2. Wastewater trickles down the media
The brown “media” layer is made of stones, gravel, plastic modules, or other high-surface-area materials.
Microorganisms grow as a biofilm on the media surface.
As wastewater slowly trickles downward:
organic pollutants adsorb onto the biofilm
microbes digest them into CO₂, water, and biomass
So the media doesn’t filter physically — it provides surface area for bacteria to attach and clean the water.
✅ 3. Air circulation
The design allows natural air flow from vents.
Air supplies oxygen so microbes stay aerobic.
Oxygen is essential because the biofilm organisms require it to break down organic matter effectively.
✅ 4. Treated water collects at the bottom
After passing through the media, the partially treated water gathers below the filter.
This is called filter effluent.
✅ 5. Final settling in a Clarifier
The effluent flows into a secondary clarifier.
Any biological solids (sloughed-off biofilm) settle as sludge.
Sludge is pumped out for sludge treatment.
✅ 6. Recycle Loop
Part of the treated water is pumped back and sprayed again.
Recycling helps:
✅ keep the biofilm moist
✅ dilute incoming wastewater
✅ improve overall efficiency
Secondary treatment: aeration
✅ How Aeration Works
Wastewater from primary treatment enters an aeration tank.
Compressed air or mechanical aerators are used to bubble oxygen throughout the tank.
Oxygen keeps microorganisms aerobic, which allows them to rapidly oxidize organic matter.
Microbes form flocs (small clumps of bacteria) that trap fine particles and digest dissolved pollutants.
The mixture of water + microbes is called mixed liquor.
What do microbes do in aeration
✅ What Microbes Do
They feed on organic material (carbon sources) in the wastewater.
Through aerobic respiration, they convert pollutants into:
✅ CO₂ (carbon dioxide)
✅ H₂O (water)
✅ new microbial biomass (sludge)As they remove the organic load, the Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) drop significantly.
What happens after aeration?
Mixed liquor flows into a final settling tank (secondary clarifier).
Microbial flocs settle at the bottom as activated sludge.
✅ Some sludge is returned to the aeration tank as Return Activated Sludge (RAS) to maintain a healthy bacteria population.
✅ Excess is removed as Waste Activated Sludge (WAS) for sludge processing.
importance of aeration
Removes 85–95% of BOD
Removes suspended solids attached to flocs
Produces high-quality effluent suitable for tertiary treatment or even discharge
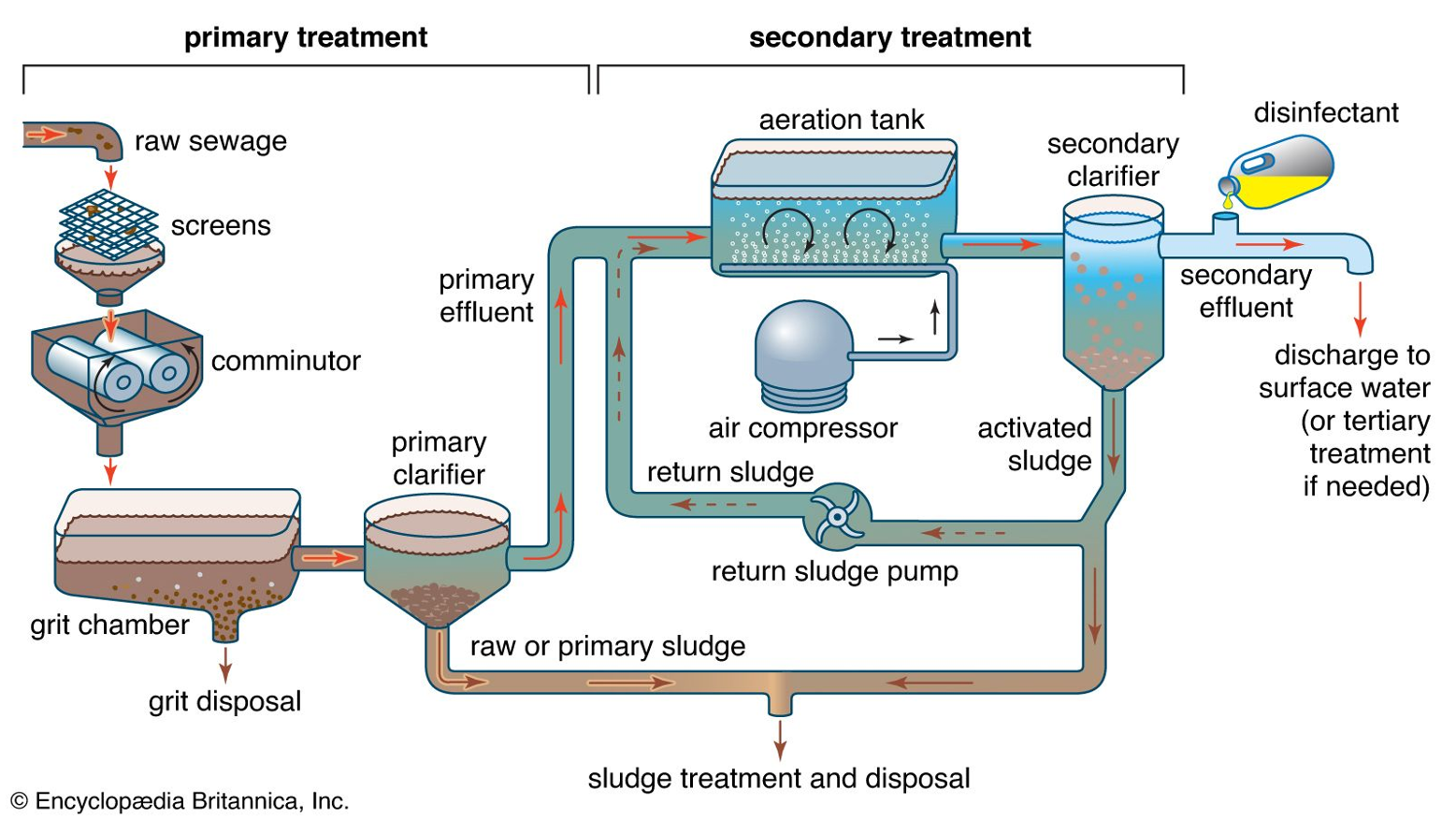
Explain this diagram (primary treatment)
1. Raw Sewage Entry
Wastewater enters the treatment plant from homes, industries, and drains.
2. Screens
Metal bar screens trap large objects such as plastics, cloth, wood, and garbage.
Removing these prevents blockages and protects machinery.
3. Comminutor
A comminutor grinds or shreds any remaining large solids into smaller pieces so they do not clog downstream equipment.
Works like a rotating grinder.
4. Grit Chamber
Wastewater slows down and heavy inorganic particles like sand, gravel, eggshells, and small stones settle.
These are removed for grit disposal.
Purpose: prevent abrasion/damage to pumps and pipes.
5. Primary Clarifier (Sedimentation Tank)
Wastewater remains still so suspended solids settle by gravity.
Bottom solids = raw or primary sludge → sent to sludge treatment.
Clear water on top = primary effluent → moves to secondary treatment.
✅ Removes about 50–60% of suspended solids and 25–35% of BOD
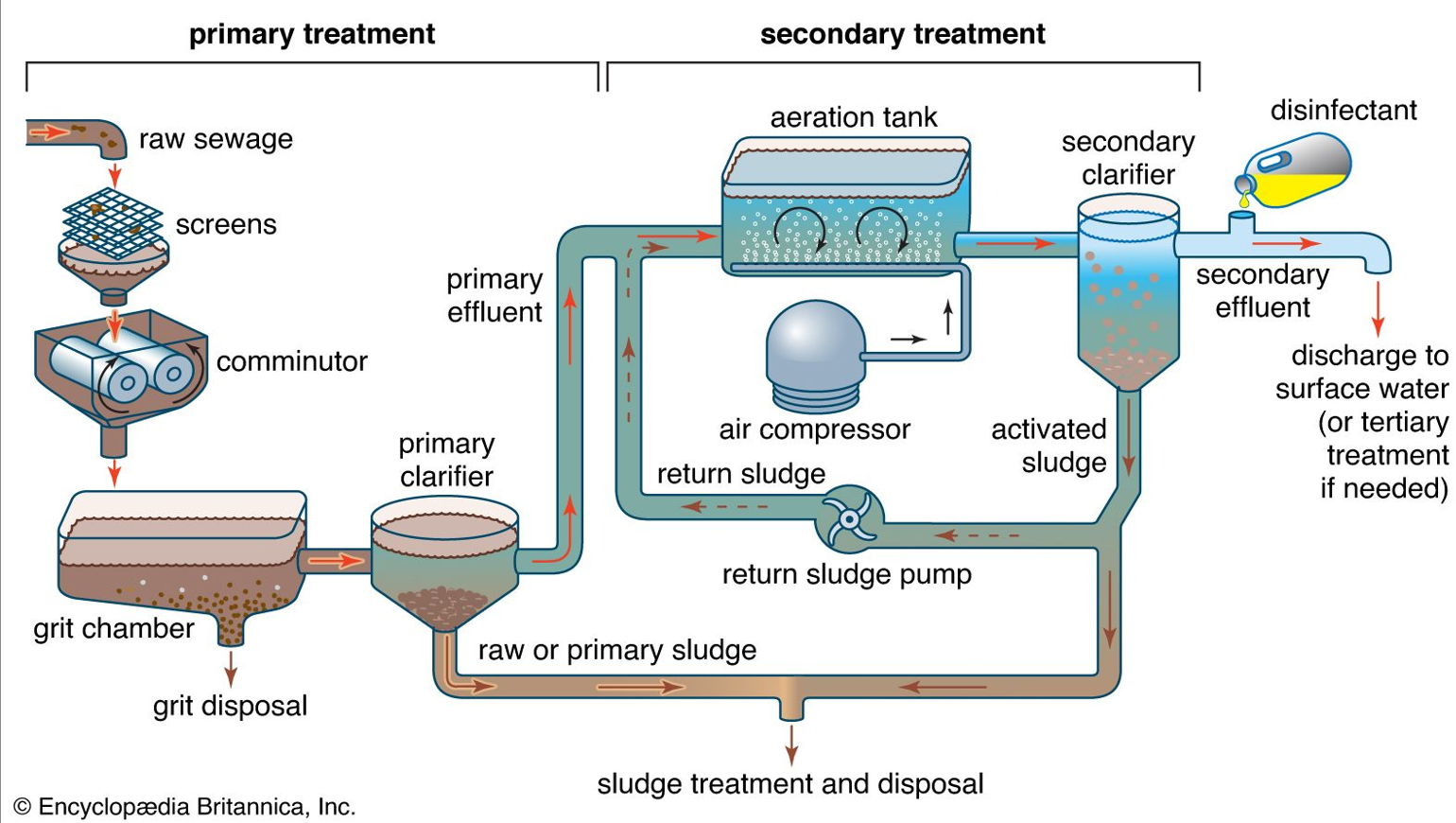
Secondary treatment: diagram
6. Aeration Tank
Primary effluent enters an aeration tank.
Air compressor pumps oxygen into the tank.
Microorganisms (activated sludge) use oxygen to break down dissolved organic pollutants into CO₂, water, and more biomass.
The mixture is called mixed liquor.
✅ Removes major portion of BOD (85–95%)
7. Secondary Clarifier
Mixed liquor flows here.
Microbial flocs settle to the bottom as activated sludge.
Clear water at the top is secondary effluent.
8. Return Activated Sludge (RAS)
A part of the settled sludge is pumped back to the aeration tank.
This keeps the bacterial population high and maintains treatment efficiency.
9. Waste Activated Sludge (WAS)
Extra sludge is removed and sent to sludge treatment and disposal.
✅ Final Steps 10. Disinfection
Chlorine, UV, or ozone kills remaining pathogens.
Makes the effluent safe before discharge.
11. Discharge
Treated water leaves the plant and is released to rivers, lakes, or oceans.
If higher quality is needed, it goes to tertiary treatment (filtration, nutrient removal, advanced disinfection).
Tertiary treatment: sand filtration
Water passes through layers of fine sand and gravel.
Sand acts as a physical filter, trapping tiny suspended particles that were not removed in sedimentation or biological treatment.
This step improves turbidity, clarity, and removes residual solids and pathogens attached to particles.
Result: a final polishing of the water before disinfection or reuse.
Tertiary treatment: adctivated carbon adsorption
Activated carbon has a very high surface area due to millions of micro-pores.
Contaminants stick to the carbon surface by adsorption.
Effective at removing:
✅ dissolved organic chemicals
✅ pesticides and industrial chemicals
✅ unwanted color, taste, and odor compoundsThis method is important for reclaimed water and situations where treated water is reused.
Tertiary treatment: chlorination
Chlorine (Cl₂), sodium hypochlorite, or chloramine are added during the final stage.
Chlorine destroys bacteria, viruses, and some protozoa by breaking down cell membranes and DNA.
Leaves a residual disinfectant in the water, meaning it continues protecting water as it travels through pipes.
Widely used because it is cheap and effective.
Tertiary treatment: UV
Water is exposed to ultraviolet light at a wavelength of ~254 nm.
UV damages the DNA of microorganisms, preventing them from reproducing.
Advantages:
✅ no chemicals added
✅ no taste or odor
✅ no harmful by-productsOften used when water is reused for irrigation or industrial uses.
Tertiary treatment: nutrient removal
✅ Nitrogen Removal
Wastewater contains nitrogen in the form of ammonia (NH₃/NH₄⁺), nitrite (NO₂⁻), and nitrate (NO₃⁻).
If released untreated, these nutrients cause algal blooms in rivers and lakes.
Treatment plants remove nitrogen using biological processes:
Nitrification – aerobic bacteria (e.g., Nitrosomonas and Nitrobacter) convert ammonia → nitrate.
Denitrification – anaerobic bacteria convert nitrate → nitrogen gas (N₂), which escapes harmlessly into the atmosphere.
Final result: much lower nitrogen entering the environment.
✅ Phosphorus Removal
Phosphorus promotes algae growth even in small concentrations.
Two main removal methods:
1. Chemical Precipitation
Chemicals like alum (aluminum sulfate), ferric chloride, or lime are added.
Phosphate reacts to form insoluble precipitates, which settle as sludge and are removed.
2. Biological Phosphorus Removal
Special bacteria called PAOs (Phosphorus Accumulating Organisms) store phosphorus inside their cells.
When these bacteria are wasted as sludge, phosphorus leaves with the biomass.
✅ Eutrophication Prevention
Nitrogen and phosphorus discharge into lakes/rivers causes rapid algae growth.
When algae die, bacteria decompose them and consume oxygen, causing:
✅ oxygen depletion (hypoxia)
✅ fish kills
✅ foul smells and toxic algal toxins
✅ disruption of aquatic ecosystemsBy removing nutrients, wastewater treatment protects water quality, fisheries, and biodiversity.
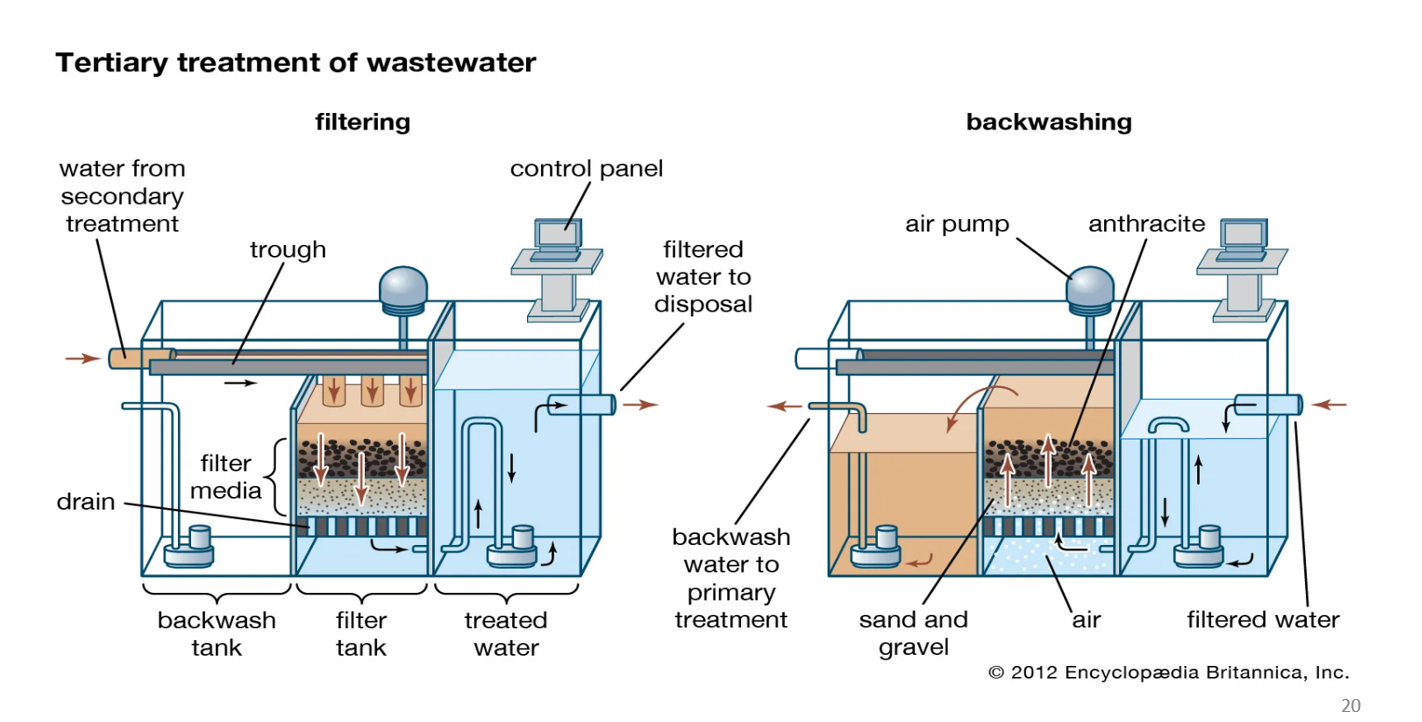
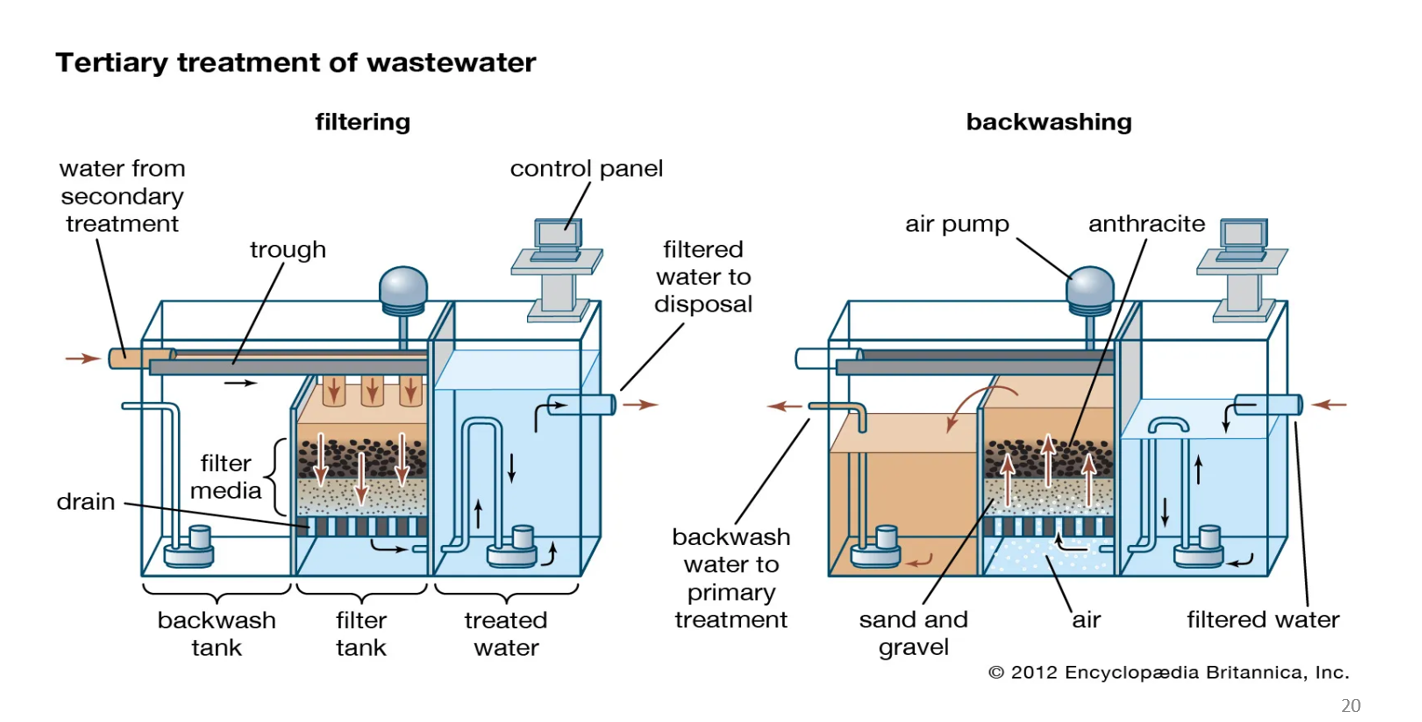
Tertiary treatment: filtering
This is the normal operation when water is being cleaned.
a. Water enters from secondary treatment
Water that has already gone through biological treatment flows into this filter tank.
b. Water moves downward through filter media
The filter bed has layers, commonly:
Anthracite (coal grains)
Sand
Gravel
As water passes down:
✅ Suspended solids get physically trapped
✅ Turbidity decreases
✅ Pathogens attached to particles are removed
This gives the water its final polish.
c. Clean water exits
The filtered water collects at the bottom and flows to the treated water tank.
From there, it is sent out as filtered water to disposal or to disinfection/reuse.
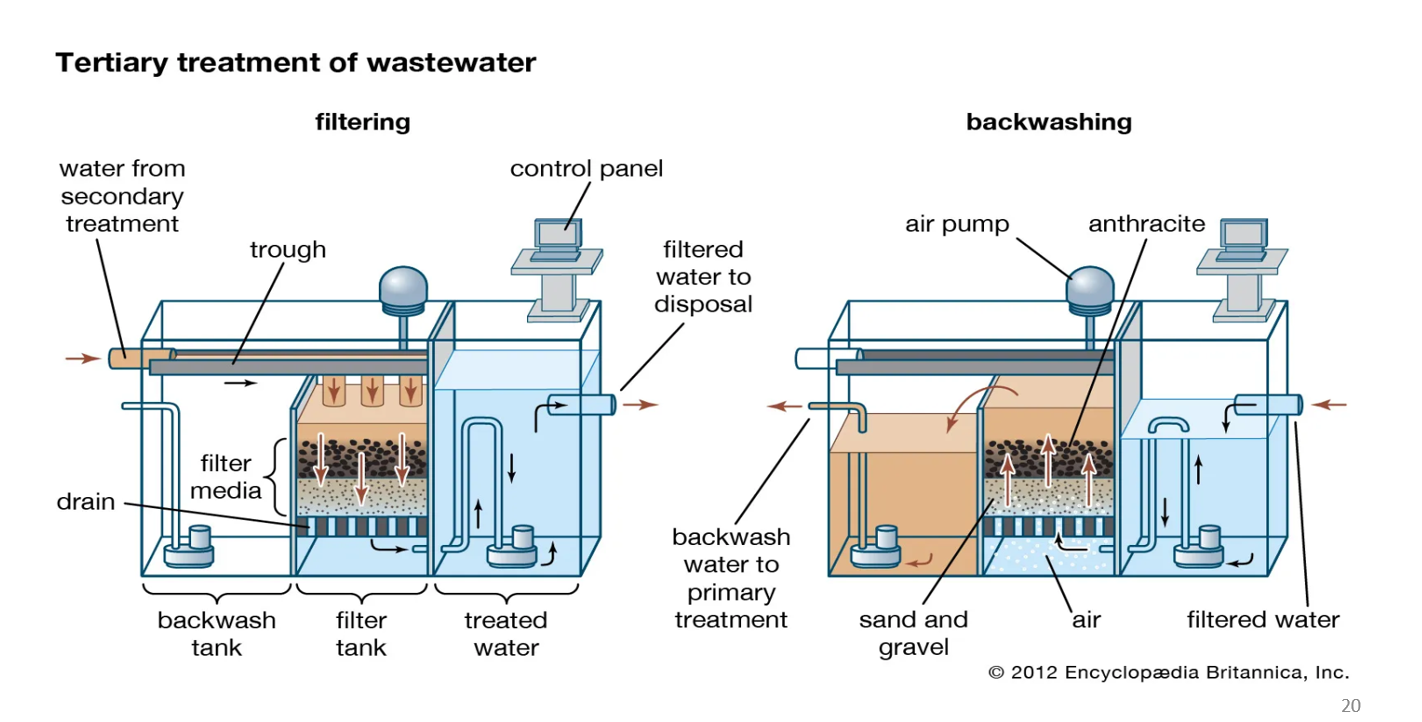
Tertiary treatment: backwashing
Over time, the filter media becomes clogged with trapped dirt.
Backwashing cleans the filter and restores flow.
a. Water flow is reversed upward
Water is pumped from the bottom and forced upward through the sand and anthracite.
The upward flow lifts and separates the filter grains, loosening trapped particles.
b. Air is added
Air pumps inject bubbles from below.
Air agitation helps shake off dirt stuck to sand grains.
This combination of air + upward water flow scrubs the filter bed.
c. Dirty water is removed
The dirty wash water (called backwash water) is collected at the top.
It flows back to primary treatment or sludge handling.
Why is backwashing needed?
✅ Why backwashing is needed
Without cleaning, the filter would clog, slow down, and stop working.
Backwashing restores:
✅ High water flow
✅ Good filtration efficiency
✅ Proper turbidity removal
Stages of sludge management
✅ 1. Thickening
Purpose: reduce the volume of sludge by removing extra water.
Methods include gravity thickening, centrifuges, or dissolved air flotation.
The solids concentration increases from ~1–2% to 4–8%, making the sludge more compact.
Thickening lowers pumping and storage costs and prepares sludge for digestion or dewatering.
✅ 2. Digestion
Thickened sludge contains biodegradable organic material.
In anaerobic digesters, bacteria break down this material in the absence of oxygen.
Benefits:
✅ Reduces pathogens and bad odors
✅ Converts organics into biogas (mostly methane + CO₂)
✅ Biogas can be used to make heat or electricity, making the plant more energy-efficientThe remaining material is called stabilized sludge because it decomposes very slowly and smells much less.
(Some plants use aerobic digestion, but anaerobic is more common for large facilities and energy recovery.)
✅ 3. Dewatering
Even after digestion, sludge still contains a lot of water.
Dewatering removes more water, turning sludge into a semi-solid cake.
Technologies: belt filter presses, centrifuges, plate-and-frame presses, drying beds.
This reduces weight and volume, making it easier and cheaper to transport or dispose.
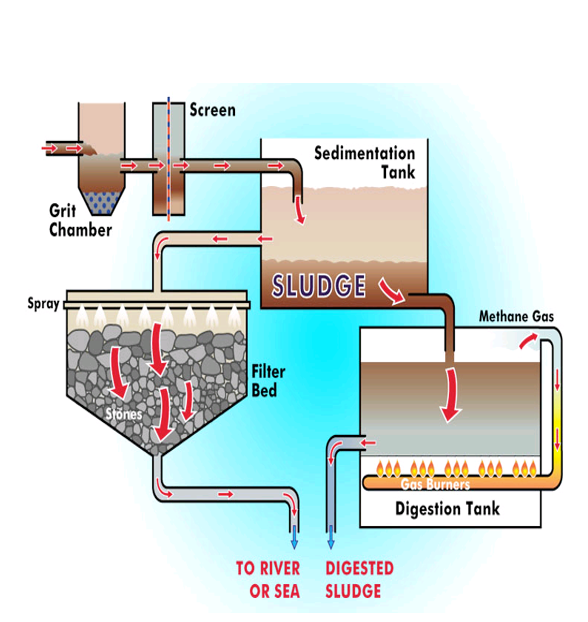
Explain this diagram
✅ 1. Grit Chamber
Incoming wastewater first enters a grit chamber.
Heavy particles like sand, gravel, and small stones settle out.
Removing grit prevents abrasion and damage to pumps and pipes later.
✅ 2. Screening
Water passes through a screen that traps larger objects: plastics, rags, wood.
These are removed and disposed separately.
✅ 3. Sedimentation Tank
Wastewater slows down in a big tank so solids can settle under gravity.
The bottom layer becomes sludge.
Clear water flows out of the tank and goes to biological treatment or straight to a filter bed (depending on the plant design).
✅ 4. Filter Bed (Trickling Filter)
Wastewater is sprayed over a bed of stones.
Microorganisms on the stone surfaces form biofilms.
As water trickles downward:
✅ organic pollutants are broken down
✅ BOD is reducedTreated water collects at the bottom and flows out to a river or sea (after final disinfection in real plants).
✅ 5. Sludge Processing
The sludge from the sedimentation tank is pumped into a digestion tank.
Digestion Tank
In this closed tank, anaerobic bacteria break down the organic matter in sludge.
This process reduces:
✅ odor
✅ pathogen levels
✅ total sludge volume
✅ 6. Methane Gas Production
Anaerobic digestion produces methane gas (biogas).
The methane is piped to gas burners.
It can be used for:
✅ heating the digester
✅ generating electricity
✅ powering plant equipment
This makes wastewater treatment more energy-efficient and eco-friendly.
✅ 7. Final Sludge
After digestion, sludge becomes stabilized.
It is easier to dewater and can be:
✅ sent to landfill
✅ used as soil conditioner (if safe)
✅ further dried/incinerated
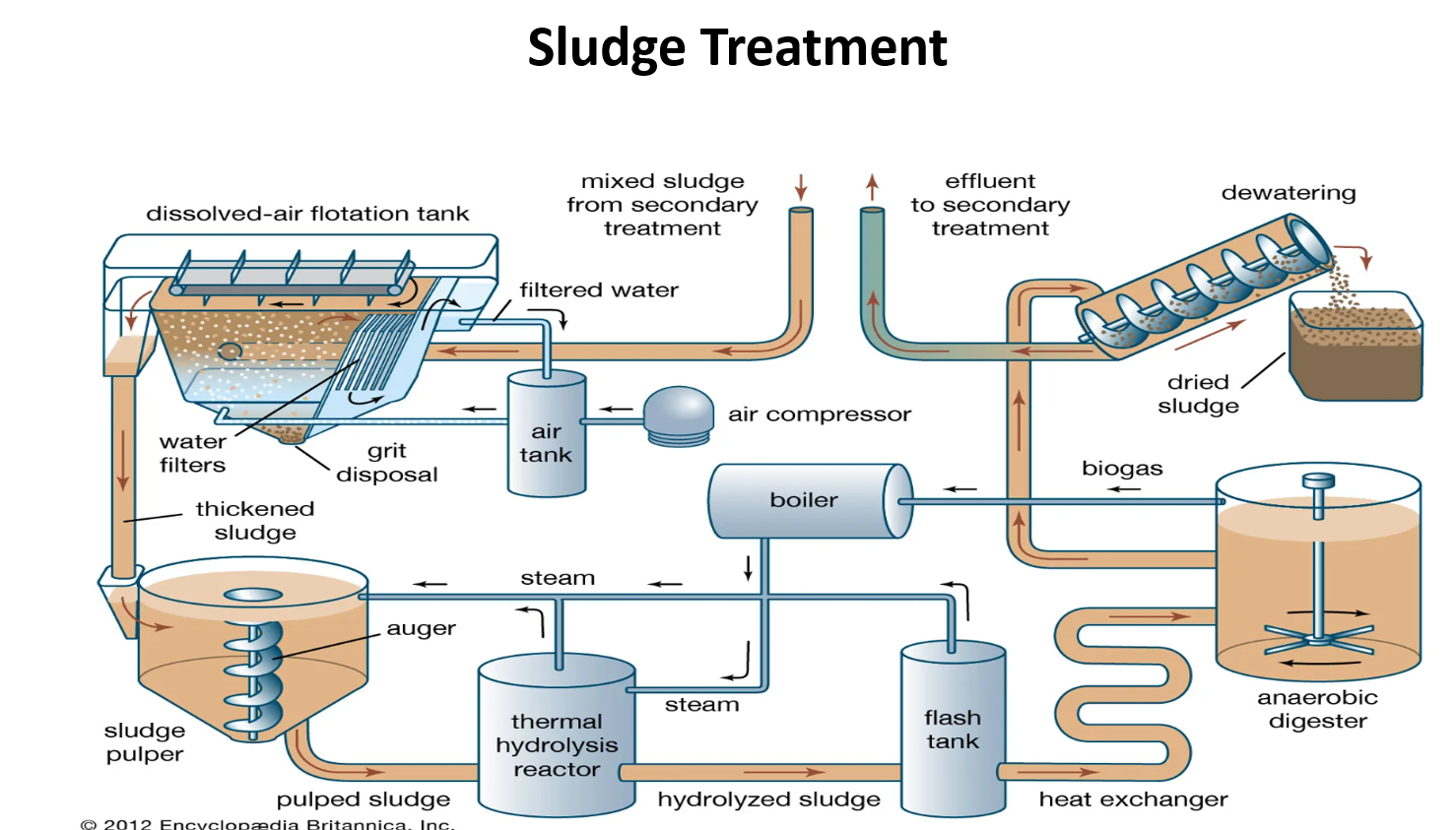
✅ 1. Dissolved-Air Flotation Tank (Sludge Thickening)
Sludge from primary and secondary treatment enters this tank.
Small air bubbles attach to sludge particles and float them to the surface.
A scraping system removes the floating sludge layer.
Result: thickened sludge (less water, more solids).
Grit and heavy particles settle below for grit disposal.
The clarified (filtered) water flows back to treatment.
✅ Purpose: reduce sludge volume before further processing.
✅ 2. Sludge Pulper
Thickened sludge goes into a sludge pulper.
An auger (rotating screw) mixes and blends the sludge.
Produces pulped sludge with uniform consistency.
This smooth sludge is easier to heat and process.
✅ 3. Thermal Hydrolysis Reactor
Pulped sludge is heated using high-pressure steam (from the boiler).
This breaks down complex organic molecules and ruptures cell walls.
Result: hydrolyzed sludge, which is easier for bacteria to digest.
Benefits:
✅ faster digestion
✅ more biogas production
✅ better pathogen destruction
✅ 4. Flash Tank
After hydrolysis, pressure is suddenly released.
This “flashing” breaks sludge particles even further and cools the sludge before digestion.
✅ 5. Anaerobic Digester
Hydrolyzed sludge enters a large closed tank with no oxygen.
Anaerobic bacteria digest organic solids.
Products:
✅ biogas (mainly methane)
✅ stabilized sludge (less odor, lower pathogen content)
Biogas is sent back to the boiler or generators.
✅ 6. Boiler + Heat Exchanger
Biogas is burned in a boiler to make steam.
Steam:
✅ heats the hydrolysis reactor
✅ keeps the digester warm (32–38°C or higher for thermophilic digestion)This recycling makes the plant energy-efficient and self-powered.
✅ 7. Dewatering
After digestion, sludge still contains water.
It goes to a dewatering unit (screw press or centrifuge).
Water is squeezed out, producing:
✅ dried sludge (solid cake)
✅ liquid effluent returned to treatment
Dried sludge is easier to handle and can be used as:
fertilizer (if safe)
landfill cover
incineration feed
Applications of sludge
1. Agricultural Fertilizer
Treated sludge (called biosolids) contains nutrients like nitrogen, phosphorus, and organic carbon.
When applied to farmland, it recycles nutrients, reduces the need for chemical fertilizers, and supports plant growth.
The organic matter improves soil fertility over time.
2. Soil Improvement
Biosolids add organic matter to soil, improving:
✅ water retention
✅ aeration
✅ soil structureThis is helpful in dry or degraded soils, allowing more water to be stored and roots to grow deeper.
3. Incineration (Energy Recovery)
Sludge can be dried and burned at high temperatures.
The heat can be used to produce steam and electricity.
After burning, only a small amount of inert ash remains, reducing waste volume.
Ash may be used in construction materials if safe.
4. Construction Materials
Treated sludge can be mixed with clay, cement, or concrete to make:
✅ bricks
✅ cement blocks
✅ roofing tilesThe organic carbon burns out during firing, leaving a lightweight, porous structure useful in building materials.
A
Decentralized and On-Site Systems - septic tanks
✅ 1. Septic Systems
A common on-site system for single homes or small buildings.
Septic Tanks
Wastewater from sinks and toilets flows into an underground, watertight tank.
Solids settle to the bottom forming sludge, while oils/grease float as scum.
Inside the tank, anaerobic bacteria partially digest organic matter, reducing odors and pathogen levels.
Liquid effluent exits the tank and goes to a drain field or another treatment step.
Purpose: Simple, low-maintenance, no electricity required.
✅ 2. Biofilters
After septic tanks, effluent can pass through a filter bed made of sand, gravel, peat, or engineered media.
Pollutants are removed by:
✅ physical filtration
✅ microbial decomposition
✅ adsorption onto media surfacesBiofilters significantly reduce BOD, suspended solids, and pathogens before water is released to soil or reused.
Common examples: sand filters, peat filters, trickling filters, compost filters.
✅ 3. Constructed Wetlands
These systems imitate the natural cleaning power of wetlands.
Wastewater flows through shallow basins filled with plants, soil, gravel, and microbial biofilm.
Plants supply oxygen to the root zone, while microbes break down organic matter and remove nutrients (nitrogen and phosphorus).
Benefits:
Low-energy and eco-friendly
Good for rural villages, parks, resorts, farms
Provide habitat for wildlife and aesthetic value
membrane bioreactor working
Biological Treatment
Wastewater enters an aeration tank.
Microorganisms break down organic pollutants, similar to the activated sludge process.
Membrane Filtration
Instead of using a secondary clarifier to settle sludge, the system uses membranes to physically separate clean water from solids.
Membranes can be:
✅ Microfiltration (0.1–0.4 µm)
✅ Ultrafiltration (0.01–0.1 µm)
Output
The filtered water (permeate) is very clear, low in pathogens and suspended solids.
Sludge remains in the tank, creating a high concentration of biomass for efficient treatment.
types of membranes in MBR
Hollow fiber modules
Flat sheet membranes
Tubular membranes
These can be placed inside the bioreactor (submerged MBR) or in a separate tank (side-stream MBR).
Benefits of MBR
Produces high-quality effluent suitable for reuse (irrigation, industrial cooling, toilets).
No secondary clarifier required, saving space.
Can operate at higher biomass concentration → smaller reactors needed.
Excellent removal of:
✅ BOD
✅ suspended solids
✅ bacteria and many virusesGood for urban areas where land is limited.
Limitations of MBR
High operating cost due to membrane cleaning and energy use.
Membrane fouling (clogging) requires regular backwashing or chemical cleaning.
More complex than conventional treatment systems.
Requires trained operators and stable electricity supply.
Whyis MBR better than activated sludge treatment
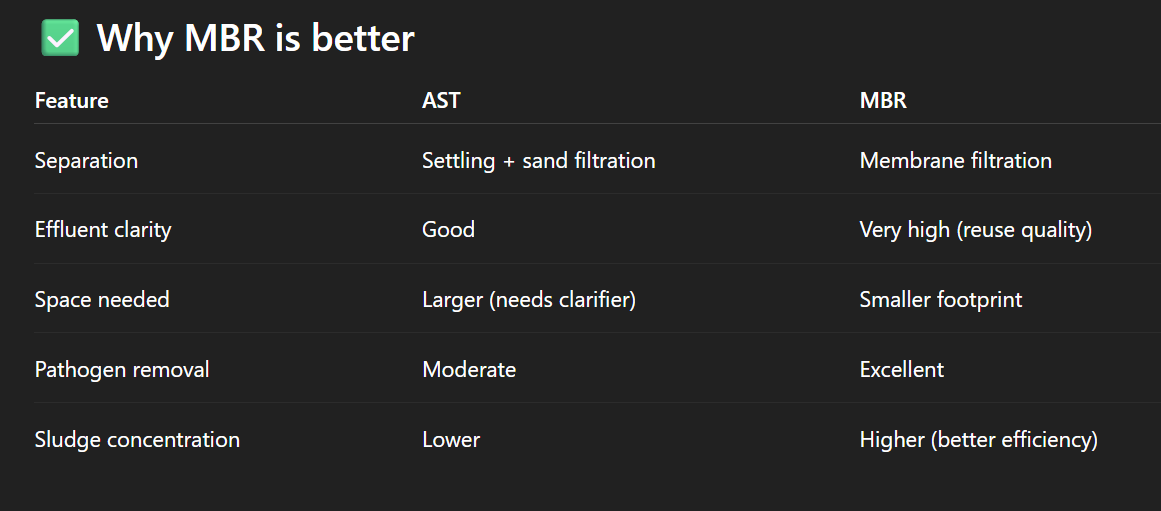
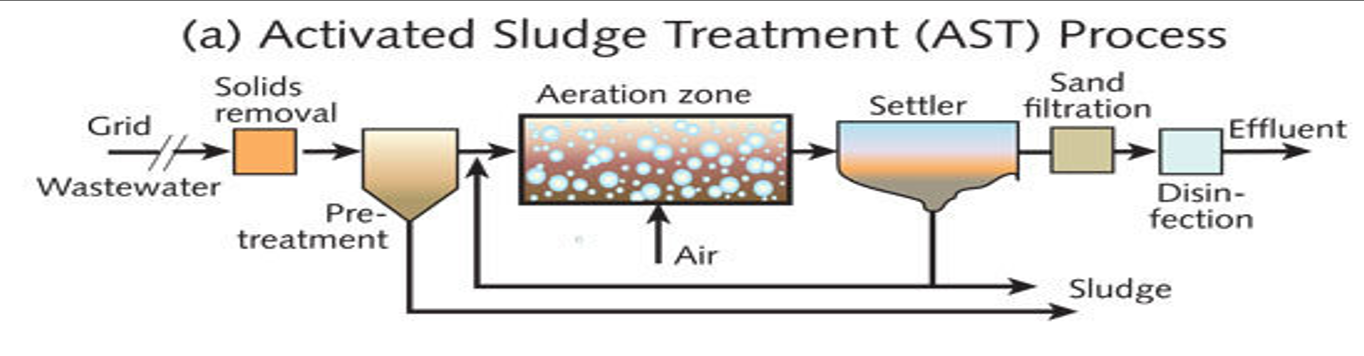
Explain AST in this diagram
Grid & solids removal
Large debris removed by screens.Pre-treatment tank
Settles heavy particles and removes grit/floating scum.Aeration zone
Air is supplied.
Microorganisms break down organic matter.
Produces mixed liquor (water + biomass).
Settler (secondary clarifier)
Biomass (activated sludge) settles by gravity.
Clear water flows out the top.
Sludge is collected at the bottom:
✅ part returned to aeration
✅ extra sent for sludge disposal
Sand filtration
Removes fine solids that escaped the clarifier.Disinfection
Chlorine or UV kills pathogens → effluent ready for discharge or reuse.
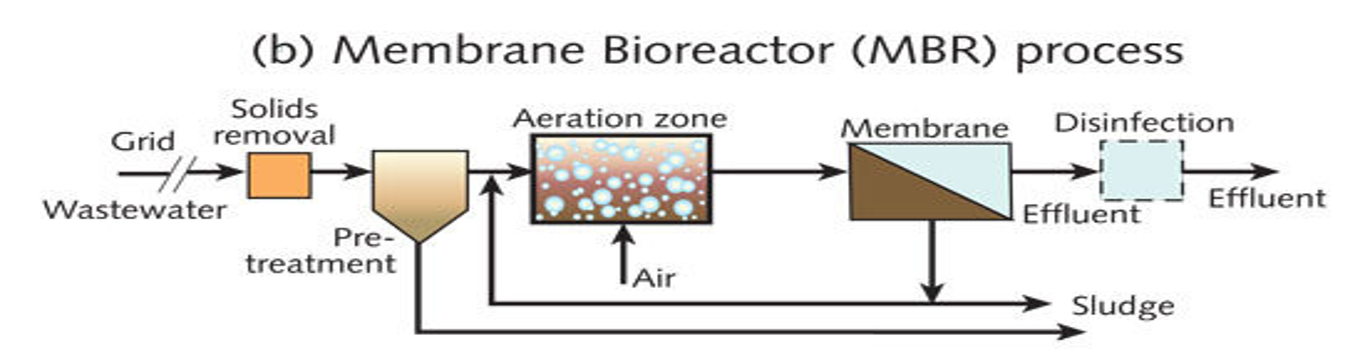
MBR treatment
Grid & solids removal
Large debris removed by screens.Pre-treatment tank
Settles heavy particles and removes grit/floating scum.Aeration zone
Air is supplied.
Microorganisms break down organic matter.
Produces mixed liquor (water + biomass).
Membrane unit
Instead of a settling tank, the system uses membranes (microfiltration or ultrafiltration).
Membranes have tiny pores that block:
✅ suspended solids
✅ bacteria
✅ many virusesOnly clean water (permeate) passes through.
Disinfection
Usually easier because membranes already remove most microbes.
Constructed wetlands
Wastewater enters a shallow basin filled with gravel, soil, or sand.
Wetland plants (like reeds, cattails, bulrushes) grow in the bed.
Microorganisms in the soil and on plant roots break down organic pollutants.
Processes involved:
✅ Filtration (solids trapped in gravel/soil)
✅ Sedimentation
✅ Microbial degradation
✅ Plant uptake of nutrients (N, P)
✅ Pathogen removal by sunlight, competition, and predationCleaned water flows out to soil, groundwater recharge, irrigation, or discharge.
types of constructed wetlands
Surface flow wetlands
Water flows above the soil surface, like a shallow pond with plants.
Subsurface flow wetlands
Water flows below the surface through gravel or sand.
Less odor and safer from mosquito breeding.
what do constructed wetlands remove?
Suspended solids
BOD and COD
Pathogens
Nitrogen and phosphorus (nutrients)
Metals and some chemicals (through adsorption and precipitation)
benefits of constructed wetlands
Low construction and operating cost
Very low energy requirement
Eco-friendly and good for wildlife habitat
Useful where centralized treatment isn’t available
Can treat sewage, greywater, agricultural runoff, landfill leachate, etc.
limitations of constructed wetlands
Requires large land area
Treatment speed is slower compared to mechanical plants
Works best in warm or temperate climates (cold slows microbes)
Needs proper design to avoid clogging or mosquito breeding
emerging trends in wastewater treatment: energy recovery
1. Energy Recovery
Modern treatment plants are shifting from energy-consuming to energy-producing facilities.
In anaerobic digesters, microorganisms break down organic sludge without oxygen.
This process generates biogas, mainly methane (CH₄).
Biogas can be:
✅ burned to produce electricity and heat
✅ upgraded to biomethane and injected into gas grids
✅ used as fuel for plant equipmentThis reduces fossil fuel use and helps plants achieve energy neutrality or even become net energy producers.
emerging trends in wastewater treatment:
Some pollutants (pharmaceuticals, pesticides, dyes, industrial chemicals) are hard to remove with conventional treatment.
AOPs generate highly reactive hydroxyl radicals (•OH) that break complex organic molecules into simpler, harmless ones.
Common AOP methods:
✅ Ozone (O₃) oxidation
✅ UV light + hydrogen peroxide (UV/H₂O₂)
✅ Ozone + hydrogen peroxide (O₃/H₂O₂)AOPs are used in hospital wastewater, industrial discharges, water reuse, and drinking water polishing.
emerging trends in wastewater treatment: nutrient recovery
Instead of just removing nitrogen and phosphorus, new systems capture and reuse them.
Phosphorus can be recovered as struvite (magnesium ammonium phosphate), a slow-release fertilizer.
Nitrogen can be recovered as ammonium salts for fertilizers or industrial use.
Benefits:
✅ reduces reliance on synthetic fertilizers
✅ prevents eutrophication
✅ turns waste into valuable resources
wastewater treatment: regulatory standards
Wastewater treatment plants must follow national and local laws that define how clean their effluent must be before it can be discharged into rivers, lakes, or oceans.
These regulations set maximum allowable levels for pollutants such as:
✅ BOD
✅ COD
✅ suspended solids
✅ nutrients (nitrogen and phosphorus)
✅ heavy metals
✅ pathogens and toxic chemicalsThese legal standards protect public health by preventing contamination of drinking water sources and reducing the spread of waterborne diseases.
They also protect the environment, ensuring wastewater does not cause oxygen depletion, algal blooms, fish deaths, or long-term ecological damage.
Governments require treatment plants to monitor and report their effluent quality regularly. Unexpected releases or poor treatment can be investigated.
Penalties for non-compliance may include:
✅ heavy fines
✅ operating permits being revoked
✅ shutdown orders
✅ legal action against the facility or organization
✅ mandatory upgrades or corrective plansStrict enforcement encourages industries and municipalities to invest in better treatment technologies and maintain proper operation.
What is the purpose of alum? (phosphorus)
✔ Chemical Phosphorus Removal
Alum = aluminum sulfate (Al₂(SO₄)₃)
When added to wastewater, alum reacts with dissolved phosphate to form insoluble aluminum phosphate.
This solid precipitates and settles out as sludge in the sedimentation tank.
Reaction:
PO₄³⁻ + Al³⁺ → AlPO₄ ↓ (solid precipitate)
This lowers phosphorus levels and prevents eutrophication in lakes and rivers.
What is the purpose of alum? (colloidal)
Wastewater often contains fine colloidal particles that do not settle naturally.
Alum causes these particles to clump together (coagulate) into larger flocs.
Flocs settle in clarifiers and can be removed easily.
Used in:
✅ primary clarification (enhanced settling)
✅ tertiary filtration systems
✅ dissolved-air flotation units
✅ water polishing before discharge or reuse
what an anthracite does
Wastewater is passed downward through a filter bed made of anthracite, sand, and gravel.
Anthracite sits on the top layer because it is lighter and has larger grain size than sand.
As water flows through it:
✅ fine suspended particles
✅ flocs
✅ leftover solids from clarifiers
are trapped between the anthracite grains.
This improves clarity and turbidity of the treated water.
why anthracite is used
High porosity → lots of empty space inside grains, excellent at holding particles
Hard and durable → does not break easily during backwashing
Large surface area for physical filtration
Works well in dual-media filters (anthracite + sand)
what are flocs
A floc is a loose, fluffy aggregate formed when fine suspended particles in wastewater coagulate and adhere to each other—often aided by chemicals like alum or by microbial activity in activated sludge.
Because flocs are larger and heavier than individual particles, they settle more easily in sedimentation tanks or get trapped in filters.
✅ Where flocs are important
Primary clarification: chemical flocculation helps solids settle
Activated sludge process: microorganisms form biological flocs that capture organic pollutants
Filtration: flocs get trapped in sand/anthracite filters
Examples of anaerobic bacteria
✅ Methanogenic (methane-producing) anaerobes
These are the key organisms in anaerobic digesters:
Methanobacterium formicicum
Methanococcus voltae
Methanosarcina barkeri
Methanosaeta concilii (very important for stable granule formation in digesters)
Methanospirillum hungatei
✅ Fermentative / Acid-forming anaerobes
Break down complex organics into acetate, H₂, CO₂, and volatile fatty acids:
Clostridium butyricum
Clostridium pasteurianum
Bacteroides fragilis
Bacteroides thetaiotaomicron
Peptostreptococcus anaerobius
✅ Sulfate-reducing anaerobes
Compete for hydrogen and produce H₂S:
Desulfovibrio desulfuricans
Desulfotomaculum nigrificans
algal blooms def
Algal blooms are rapid, excessive growths of algae in water bodies (lakes, rivers, ponds, coastal waters) caused mainly by high nutrient levels, especially nitrogen and phosphorus.
causes of algal blooms
When wastewater or agricultural runoff adds too much N and P to water, algae multiply very quickly.
Warm temperatures, sunlight, and stagnant water make the growth even faster.
Why algal blooms are dangerous
Oxygen depletion (hypoxia):
When algae die and decompose, bacteria consume dissolved oxygen, leading to fish kills.Eutrophication:
Excessive nutrient enrichment and plant growth that degrade water quality.Toxin production:
Certain species (e.g., Microcystis aeruginosa, Anabaena flos-aquae) release cyanotoxins harmful to fish, livestock, and humans.Reduced light penetration:
Dense algal mats block sunlight, killing aquatic plants and disrupting ecosystems.Foul odor and taste:
Water becomes smelly and unpleasant, making it unfit for recreation or drinking.
anaerobic digestion of sludge
Wastewater sludge goes into an airtight chamber (digester).
Anaerobic bacteria break down the organic matter without oxygen.
This stabilizes sludge by:
✅ reducing its volume
✅ lowering pathogens
✅ reducing foul odorA major byproduct is methane gas (biogas).
methane capture
The methane produced in the digester is collected.
It can be used as a renewable energy source.
Examples:
✅ fuel for boilers
✅ powering generators
✅ heating the digester itself
This reduces reliance on fossil fuels.
sludge pre-treatment
Sludge can be pretreated chemically (like adding 2M NaOH) or physically (heating, grinding) to:
✅ increase biodegradability
✅ increase methane production
✅ make organic matter more easily available to microbes
So, microbes convert sludge to methane faster and more efficiently.
conditions for
Temperature: 35–37°C (mesophilic digestion)
pH: near neutral (around 7)
These conditions allow anaerobic bacteria to perform best.
final use of sludge
After digestion and drying, sludge becomes safe to handle.
✅ Soil conditioner / fertilizer
Adds organic matter and nutrients to soil.
Dried sludge can improve soil structure.
✅ Construction materials
Used in cement industry, brick making, or composting.
Chemical effluents
Acids: Sulfuric acid from fertilizer plants; nitric acid from explosives manufacturing; hydrochloric acid from metal pickling.
Alkalis: Sodium hydroxide from soap/detergent factories; calcium hydroxide from tanneries; ammonia from fertilizer plants.
Heavy metal effluents
Lead: Battery manufacturing and recycling units.
Mercury: Chlor-alkali plants, fluorescent lamp production.
Biodegradable effluents
Food-processing waste: Sugar mill wastewater rich in molasses; dairy plant wastewater containing fats and proteins.
Agricultural waste: Runoff containing manure, crop residues, and plant biomass.
Thermal effluents
Power plants: Discharge of 40–60°C condenser cooling water into rivers.
Steel mills: Hot quenching water from coke ovens.
Radioactive effluents
Nuclear power plants: Tritium-containing wastewater; cesium-137 and strontium-90 trace releases.
Medical facilities: Iodine-131 from radiotherapy units; technetium-99m from diagnostic imaging
pharma effluents
Antibiotics: Ciprofloxacin, amoxicillin, erythromycin from pharmaceutical plants and hospital wastewater.
Hormones: Estrogens (EE2, E2) from contraceptive manufacturing and hospital discharge
Physical parameters of waste water
Temperature
Indicates heat content of wastewater; affects reaction rates and dissolved oxygen levels.Color
Caused by dyes, humic substances, industrial chemicals; indicates organic load or industrial contamination.Odor
Suggests anaerobic decomposition (rotten egg smell from H₂S), presence of chemicals, or sewage putrefaction.Turbidity
Measure of suspended particles; affects light penetration and treatment efficiency.Total Suspended Solids (TSS)
Solid particles >2 µm that remain undissolved; high TSS indicates poor primary treatment.Total Dissolved Solids (TDS)
Salts, minerals, and low-molecular-weight organics dissolved in water.Conductivity
Indicates ionic strength; higher conductivity = higher dissolved salts.Settleable Solids
Particles that settle within 1 hour (Imhoff cone); important for sedimentation design.
Chemical parameters of waste water
Dissolved Oxygen (DO)
Oxygen dissolved in water; indicates aeration and ecological health.Biochemical Oxygen Demand (BOD₅)
Amount of oxygen required by microorganisms to degrade organic matter over 5 days.Chemical Oxygen Demand (COD)
Total oxygen required to oxidize organic and inorganic matter chemically.Total Nitrogen (TN)
Includes nitrate, nitrite, ammonia, organic nitrogen.Ammonia (NH₃/NH₄⁺)
Indicator of sewage pollution and protein degradation.Nitrate (NO₃⁻) and Nitrite (NO₂⁻)
Indicators of nitrification/denitrification processes.Total Phosphorus (TP)
Includes phosphates; contributes to eutrophication.
Textile & Dyeing Industry: top industries generating effluents
Why it generates high effluents:
Requires large volumes of water for washing, scouring, bleaching, dyeing, and rinsing.
Uses synthetic dyes, salts, alkalis, surfactants, which enter wastewater.
Frequent batch processes mean continuous discharge.
Effluent is highly colored, alkaline, and chemically complex.
Pulp & Paper Industry: top industries generating eff
Huge water requirement for pulping, washing, bleaching, and paper formation (tons of water per ton of paper).
Produces lignin-rich, high-BOD/COD wastewater.
Bleaching releases chlorinated organics, making treatment difficult.
Mechanical operations continuously wash fibers, generating large flow rates.
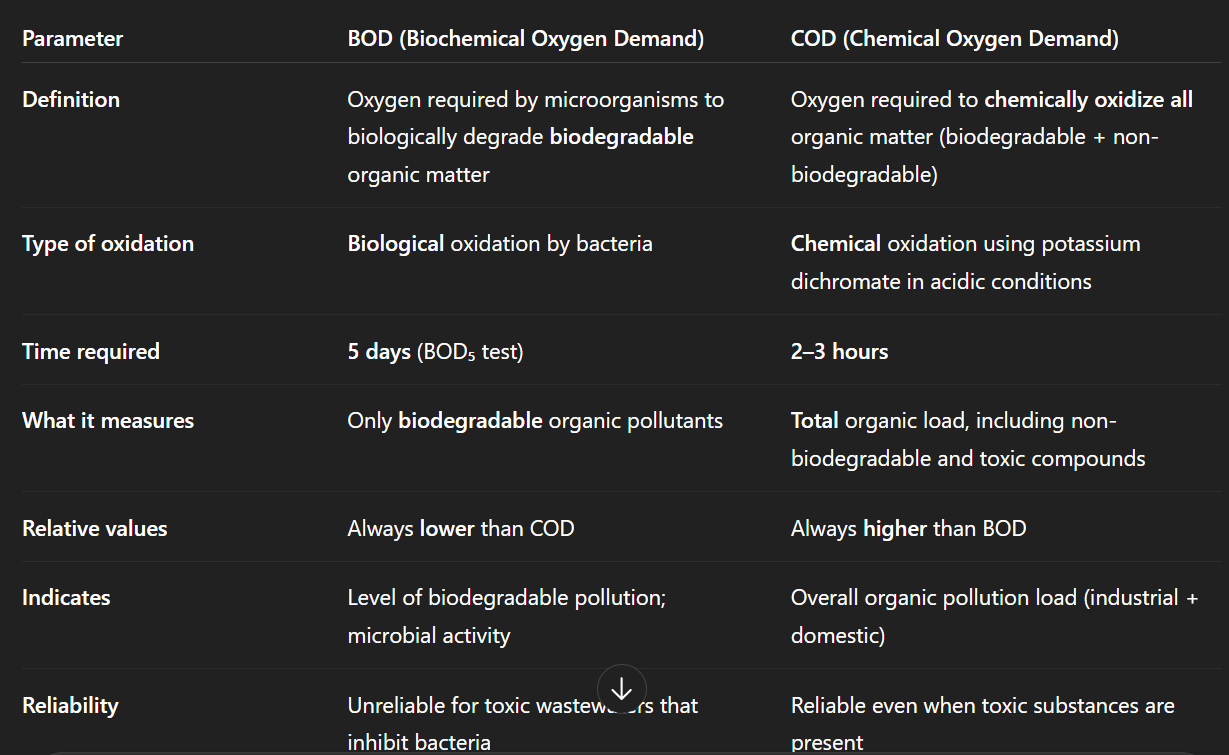
Difference between COD and BOD
Textile waste water contains (pH, COD, BOD, dyes)
• very high pH (5–12)
• very high COD (up to 2250 mg/L)
• very high BOD (up to 3000 mg/L)
• dyes (10–800 mg/L)
Textile pre-treatment: screening
What it removes:
• cloth pieces
• lint
• threads
• buttons
• packaging residues
• undissolved solids
Why?
Because these block pumps, pipes, filters, and aeration systems.
How?
Wastewater passes through steel bars or mesh screens.
Think of this like a “sieve” for wastewater.
Textile pretreatment: sedimentation
Removes:
• sand
• soil
• small stones
• metal particles from machinery
Why?
Heavy particles damage pumps and create sludge buildup.
Textile pre-treatment: equalization
Textile factories produce different wastewater every minute:
• bleaching water (very alkaline)
• dye bath water (very colored)
• wash water (dilute)
The equalization tank mixes everything to make:
• uniform pH
• uniform color
• uniform pollutant levels
• steady flow rate
Why?
Biological systems cannot handle sudden shocks like pH 12 then pH 4.
This step protects the entire treatment plant.
Textile primary treatment: neutralization
What’s the problem?
Textile wastewater pH can be 5 to 12 (slide data).
Microbes only live at pH 6.5–8.
How it’s fixed:
• Add H₂SO₄ or HCl to reduce high pH.
• Add lime or NaOH to raise low pH.
Why it matters:
If you skip neutralization → secondary treatment dies immediately.
Textile primary treatment: coagulation
Textile dyes and colloids are too small to settle.
They are negatively charged and repel each other → stay floating forever.
Solution:
Add coagulants like:
• FeCl₃ (ferric chloride)
• Al₂(SO₄)₃ (alum)
These neutralize the charges.
Result:
Particles can now come together.Textile
Textile primary treatment: flocculation
After coagulation, particles still need help forming “flocs.”
How it works:
• Add polymers (flocculants) or use slow mechanical paddles
• Particles collide and form larger aggregates
Why?
Big particles settle → small ones don’t.
Your lecture’s example:
Cactus mucilage is a natural flocculant that works surprisingly well.
It binds particles efficiently and reduces color, COD, and turbidity.
Textile primary treatment: sedimentation
Process:
• Wastewater enters a settling tank
• Heavy flocs fall to the bottom as sludge
• Cleaner water flows out from the top
Slide notes:
Sludge is mechanically scraped and pumped out.
Why this step matters:
• Removes 50–70% of suspended solids
• Reduces 20–30% of BOD
• Reduces 30–60% of turbidity
• Removes a lot of color and metals
This is the final step before biological treatment.
Purpose of GO
Graphene oxide is a single-layer carbon material with:
✔ a huge surface area
✔ many oxygen-containing groups (–OH, –COOH, epoxides)
✔ very strong adsorption ability
✔ high reactivity with dyes
Textile wastewater contains high dye concentrations, especially azo dyes. These dyes are difficult to remove using normal coagulation and flocculation because they are:
• highly soluble
• strongly colored
• chemically stable
• small molecules that don’t settle easily
How Graphene Oxide removes color
1. Adsorption
GO has a massive surface area (~700–1500 m²/g).
Dye molecules “stick” to its surface like velcro.
This alone removes a huge amount of color.
2. π–π interactions (Carbon rings binding dye rings)
Most dyes contain aromatic rings.
GO also contains aromatic carbon rings.
These interact strongly → trapping dye molecules.
3. Electrostatic attraction
GO has oxygen groups that make it negatively charged.
Many dyes are positively charged (cationic dyes).
Opposite charges attract → dye sticks to GO.
Secondary treatment of textiles: aerobic biological treatment
Heterotrophic bacteria oxidize organic pollutants using O₂ as the terminal electron acceptor
Energy released supports:
• cell synthesis
• enzyme production
• nutrient uptake
Secondary treatment of textiles: aerobic biological treatment kinetics

Secondary treatment of textiles: aerobic biological treatment optimal conditions
DO: 2–4 mg/L
pH: 6.5–8.0
Temperature: 20–35°C
HRT: 12–48 hours (depending on system)
Sludge age: 5–15 days
Secondary treatment of textiles: aerobic biological treatment
Systems used
✔ Aerated lagoons – large basins with surface aerators
✔ Activated sludge (implied though not named in slides)
✔ Trickling filters – attached biofilm reactors
Secondary treatment of textiles: aerobic biological treatment
trickling filters mechanism
A biofilm forms on stones/plastic media. As wastewater trickles:
Mass transfer delivers pollutants into the biofilm.
Aerobic layer on the outside oxidizes organics.
Anoxic inner layer helps denitrification.
Typical organic load: 0.08–0.4 kg BOD/m³·day
Secondary treatment of textiles: aerobic biological treatment
dye removal in systems
Aerobic systems remove dyes mainly by:
• partial oxidative cleavage
• co-metabolism with other organics
• biosorption onto biomass
• enzymatic transformation (less effective on azo dyes)
Limitation:
Azo dyes resist aerobic breakdown because the N=N bond requires a reductive (anaerobic) environment to cleave.