S1.2 - Electrophiles
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
44 Terms
What are acids?
Proton donors
How do acids dissociate?
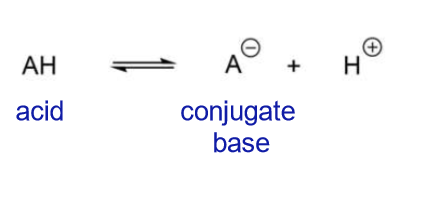
How is acidity rationalised?
Depends on resonance and inductive effects
What does dissociation of a carboxylic acid result in?
Breaking the O-H bond results in the formation of an oxygen anion and a proton
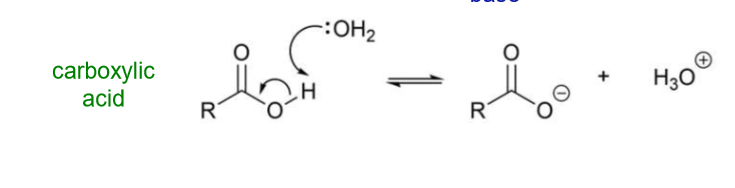
How is a hydronium ion generated?
In water, the proton lost is bonded to a water molecule
What is the acid dissociation constant?
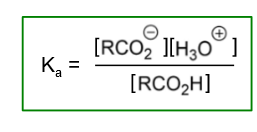
How can pKa be measured?
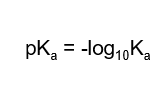
How can pKa be interpretted?
As the pH of 50% dissociation
What does a lower pKa mean?
More acidic
How does stabilising an anion (negative charge) effect acidity?
Stabilising an anion will move the equilibrium to the RHS and enhance the acidity of a species as it is more likely to give up a proton
How do amines dissociate?
The conjugate ammonium ion dissocates
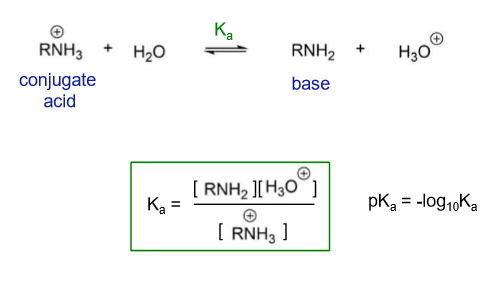
What is the pKa of amine?
10-11
What makes the base more basic?
Depending on whether it is primary, secondary or tertiary
Tertiary makes the conjugate acid more stable
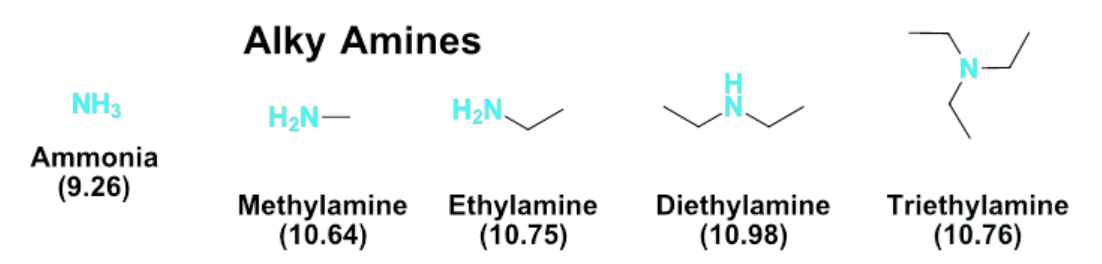
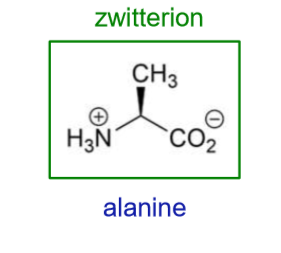
What is a zwitterion?
neutral species that exists with both positive and negative charges
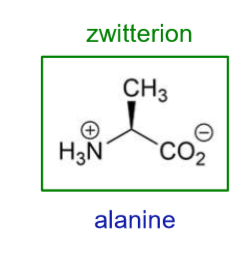
What is the pKa for RCO2H?
2.34
What is the pKa for RNH3?
9.69
What is the isoelectric point?
the pH at which there is no net charge
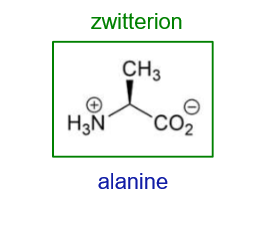
What will happen to alanine at pH 1?
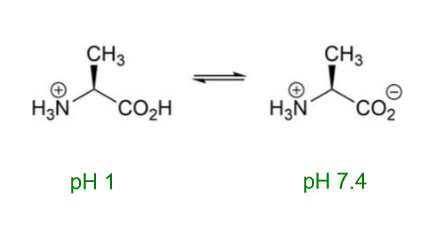
What will happen to alanine at pH 13?
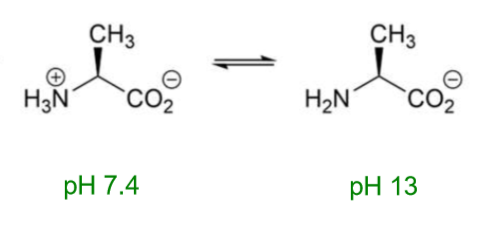
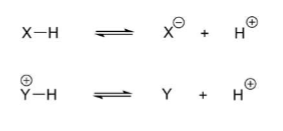
What does acidity depend on?
Bond strength of X-Y or Y+ -H (weaker bonds more easily broken)
Solvation (assume in water as it is good at solvating polar molecules)
Whether the charge of the cation or anion can be stabilised
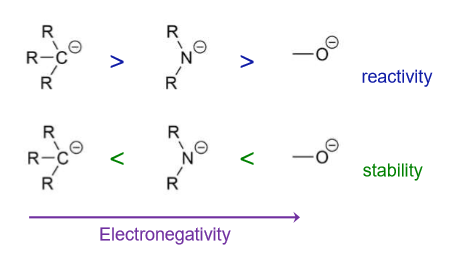
How does electronegativity effect reactivity and stability?
Molecules containing more electronegative atoms will produce more stable anions
Substituents also make a difference
Why are side chains important on amino acid residues?
For determining overall charge of a protein
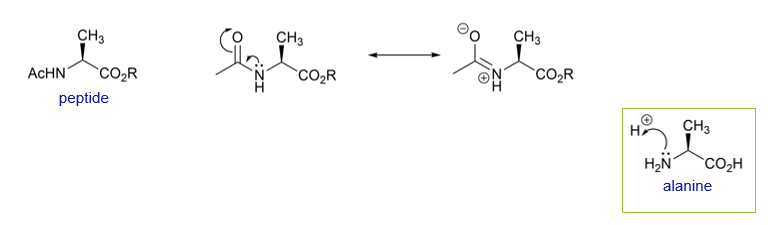
How does alanine change when in isolated form vs part of a peptide chain?
Alanine can be easily protonated In its isolated form, but when part of a peptide chain, with amide bonds at the amine and acid functionalities, the charge is distributed differently
The nucleophilicity of the lone pair on N is now less as a result of the resonance stabilisation afforded by the carbonyl adjacent to the N
Why are properties of amino acids different when they are within a peptide chain to when they are alone or at the ends of chains?
Carboxylic acids can dissociate but amines or esters cannot
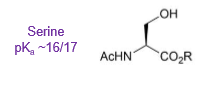
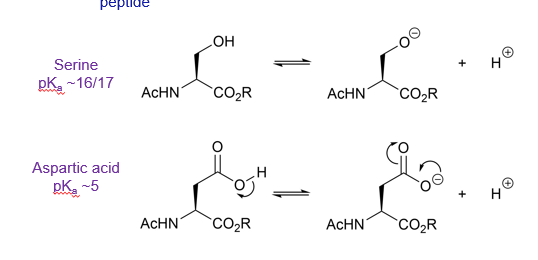
What is the pKa of serine?
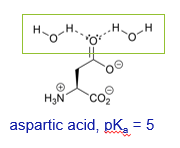
What is the pKa of aspartic acid?
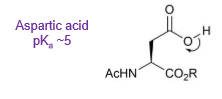
Why is the side chain of aspartic acid more acidic than the side chain of serine?
The anion resulting from deprotonation can be delocalised and therefore more stable
Delocalisation of the charge means the molecule is more stable so its formation is more favourable and the pKa will be lower. Making it a stronger acid
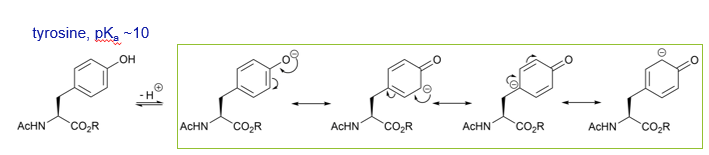
What is the pKa of tyrosine?
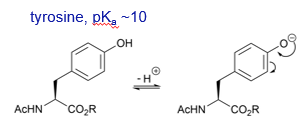
Why is tyrosine more acidic than serine?
The side chain of tyrosine is also more acidic than the side chain or serine as the anion resulting from deprotonation can be delocalised
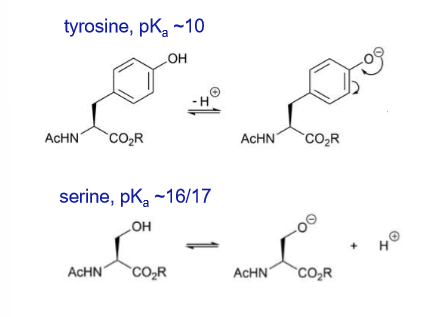
What are the resonance forms of tyrosine?
Why is the side chain of aspartic acid more acidic than tyrosine?
The anion resulting from deprotonation is stabilised greater through solvation with water molecules
What is acidity a measure of?
The stability of the conjugate base, the more stable the anion, the less likely it is to undergo reaction with a proton or with a positively polarized carbon atom
What does an increased basicity equate to?
A more reactive source of electrons to accept protons.
The higher pKa of conjugate acid, the more reactive the base
What is an electrophile?
A reagent that possess an atom that is electron deficient
Can accept an electron pair from a second molecule of higher electron density
What are examples of electrophiles?
Eg protons, metal cations, electron deficient carbon atoms and other electron poor atoms
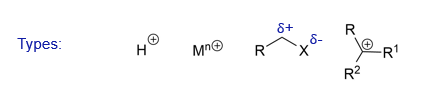
Which electrophiles and more reactive?
Those with full positive charge are more reactive than those with a partial positive charge (small degree of polarization)
Why are unstabilised positive charges centered on carbons not often seen in biological systems?
They are energetically unfavourable
How is stabilisation of carbocations achieved?
Through inductive effects or resonance eg using adjacent oxygen lone pairs or aromatic rings
Why are alkyl halides with positively polarized carbons cytotoxic?
they can undergo reactions with biological molecules within cells
What is hyperconjugation?
A greater number of alkyl groups bonded to the carbon bearing a positive charge results in increased stability.
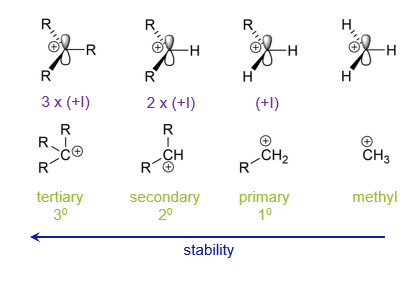
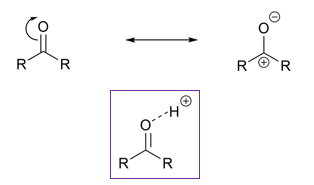
What are carbonyls?
What are imines?
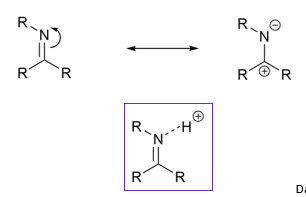
Why are carbonyls and imines electrophillic?
Due to resonance
How can carbonyls and imines be made more reactive?
Protonated
OR coordinated with metal ions