Polymers
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Most polymer chains have crystalline and amorphous regions
true
Amorphous regions
molecules arranged randomly giving the polymer more flexibility and contains Van der Waals and H-bonding
Crystalline regions
Molecules in highly ordered position to make polymers more rigid and contains hydrogen and dipole-dipole bonding
Branched Polymers
Polymers with side chains off the main chain, causing them to be amorphous, soft, and low density, since branching prevents chains from packing closely
-IMFs: Van der waals
Cross-linked (network) polymer
A type of polymer where the individual chains are interconnected through branches or disulfide bridges in between.
IMFs: Hydroden bonds, Van der waals, and dipole-dipole
Linear polymers
polymers that consist of long, straight chains of monomers, allowing for more efficient packing and increased crystallinity.
IMFs: Van der waals and H bonds
How does branching affect the density of olefins?
As branching increases, density of olefins decrease, van der waals forces become weaker and the polymer becomes more amorphous due to the distance between molecules.
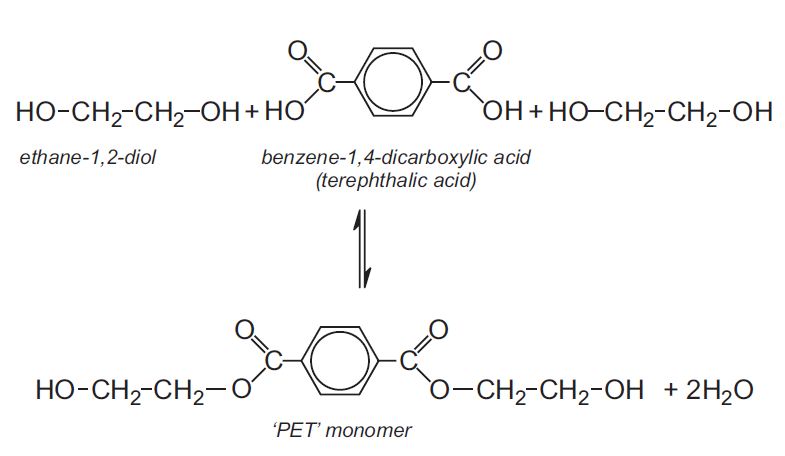
polyester
A category of polymers formed from the reaction between an alcohol and a carboxylic acid, characterized by ester linkages. Commonly used in textiles and plastics.
-Ex: Polyethylene Terephthalate (PET)
polyurrethane