Types of equilibria + Kc
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What does it mean if an equilibria is homogeneous?

What does it mean if an equilibria is heterogeneous?

Which state of products and reactants are included in the Kc equation?
Only (g) and (aq)
Why are solids and liquids omitted in Kc?

How do calculate Kc of a homogenous equilibria?
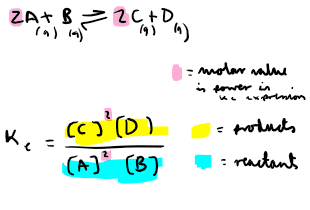
How do calculate Kc of a heterogenous equilibria?
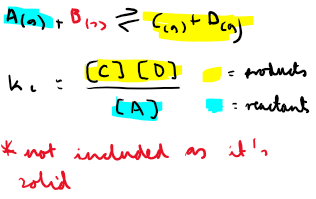
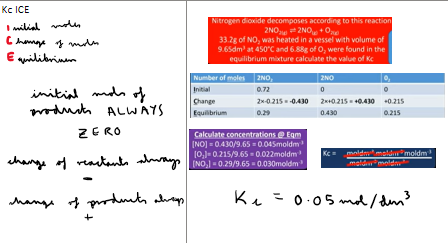
How do you use ICE to calculate Kc?
What factors affect Kc?
Temperature
Concentration of reactants
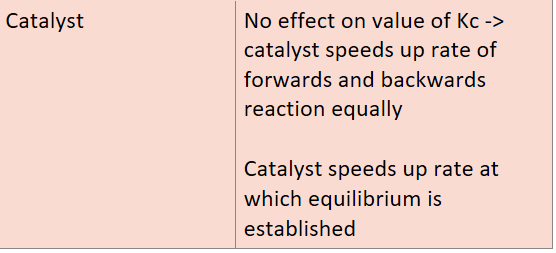
Catalyst
How does the position of the equilibrium affect Kc?
MAIN PRINCIPLE-
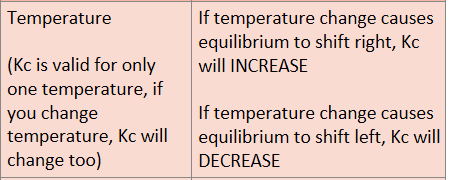
Equilibrium shifts to right: Kc value increases
Equilibrium shifts to left Kc value decreases
How does temperature affect Kc?
How does concentration of reactants affect Kc?

How does a catalyst affect Kc?
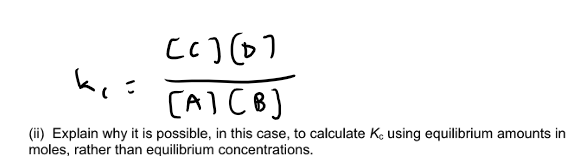
Same number of moles on both side of equation, so volume cancels out.