Chemistry Final
5.0(1)
Card Sorting
1/97
Last updated 1:31 AM on 6/12/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
98 Terms
1
New cards
6
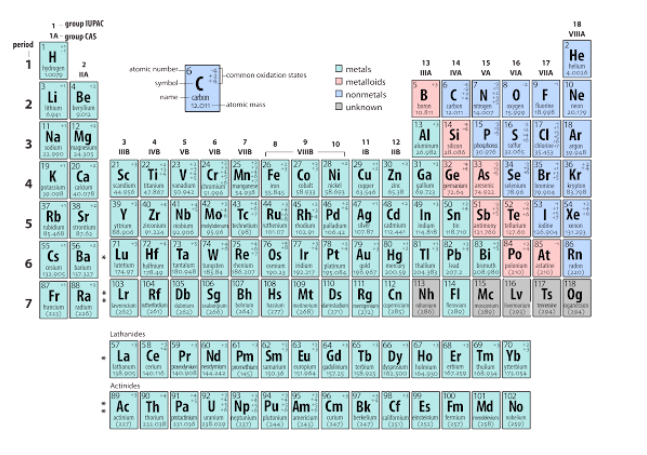
How many electrons are being shared by the atoms in the model below?
\
1. 2
2. 4
3. 6
4. 8
\
1. 2
2. 4
3. 6
4. 8
2
New cards
b
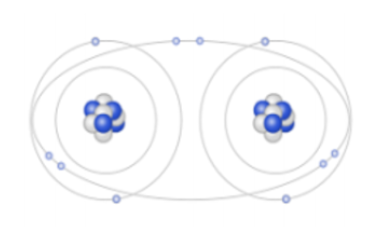
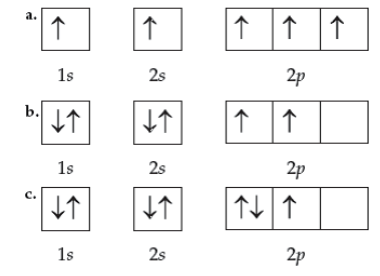
Which orbital diagram is done correctly?
\
1. a
2. b
3. c
\
1. a
2. b
3. c
3
New cards
electronegative
An atom that does not share electrons equally in a covalent bond and pulls the electrons to itself is described as
1. electronegative
2. endothermic
3. exothermic
4. electropositive
1. electronegative
2. endothermic
3. exothermic
4. electropositive
4
New cards
4 grams
Consider the following chemical reaction: Hydrogen + Oxygen combine to form Water. If 36 grams of water are made and 32 grams of Oxygen are used, how many grams of Hydrogen are used?
\
1. 40 grams
2. 4 grams
3. 68 grams
4. 14 grams
\
1. 40 grams
2. 4 grams
3. 68 grams
4. 14 grams
5
New cards
Because they have a full set of valence electrons (and are stable)
Why do noble gases generally not form chemical bonds with other atoms?
\
1. Because they are nonmetals
2. Because they only form bonds with each other
3. Because they have a full set of valence electrons
4. Because they are unreactive
\
1. Because they are nonmetals
2. Because they only form bonds with each other
3. Because they have a full set of valence electrons
4. Because they are unreactive
6
New cards
Sugar dissolves in water
Check the box that describes a physical change in matter
\
1. Sodium metal catches on fire when placed in water
2. Sugar dissolves in water
3. Hydrochloric acid reacts with potassium hydroxide to produces salt, water, and heat.
4. Silver jewelry tarnishes over time
\
1. Sodium metal catches on fire when placed in water
2. Sugar dissolves in water
3. Hydrochloric acid reacts with potassium hydroxide to produces salt, water, and heat.
4. Silver jewelry tarnishes over time
7
New cards
Carbon
Which of the following elements can form 4 covalent bonds?
1. Hydrogen
2. Carbon
3. Nitrogen
4. Oxygen
1. Hydrogen
2. Carbon
3. Nitrogen
4. Oxygen
8
New cards
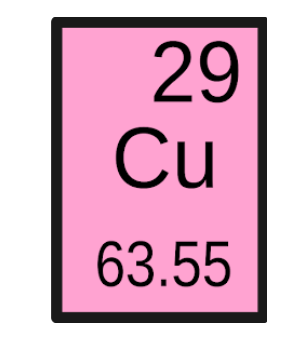
138\.539 grams
Using the information given below for Copper, calculate the mass of 2.18 moles of copper. Please choose the answer that you calculate exactly, do not round.
\
1. 138.53 grams
2. 138.539 grams
3. 63.22 grams
4. 138.53028 grams
\
1. 138.53 grams
2. 138.539 grams
3. 63.22 grams
4. 138.53028 grams
9
New cards
atoms tend to gain, lose or share electrons in order to acquire a full set of 8 valence electrons.
The Octet Rule states that __________________
\
1. atoms are always found in groups of 8.
2. the Periodic Table has 8 groups.
3. all electron orbitals can fit exactly 8 electrons.
4. atoms tend to gain, lose or share electrons in order to acquire a full set of 8 valence electrons.
\
1. atoms are always found in groups of 8.
2. the Periodic Table has 8 groups.
3. all electron orbitals can fit exactly 8 electrons.
4. atoms tend to gain, lose or share electrons in order to acquire a full set of 8 valence electrons.
10
New cards
compound
H2O is a/an
\
1. heterogeneous mixture
2. homogeneous mixture
3. element
4. compound
\
1. heterogeneous mixture
2. homogeneous mixture
3. element
4. compound
11
New cards
14
How many Oxygen atoms are in the reactants below?
\
1. 6
2. 8
3. 14
4. 2
\
1. 6
2. 8
3. 14
4. 2
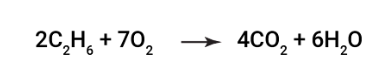
12
New cards
covalent
What type of bond will form between two oxygen atoms?
1. covalent
2. metallic
3. ionic
4. polar covalent
1. covalent
2. metallic
3. ionic
4. polar covalent
13
New cards
oxygen
Which of the following is an element:
\
1. water
2. air
3. oxygen
4. fire
\
1. water
2. air
3. oxygen
4. fire
14
New cards
p block
Groups 13-18, with the exception of He, make up the
\
1. d block
2. p block
3. s block
4. f block
\
1. d block
2. p block
3. s block
4. f block
15
New cards
GROUPS !!!!!!!!
What are the vertical columns on the periodic table called?
\
1. periods
2. groups
3. noble gases
4. Lanthanides and Actinides
\
1. periods
2. groups
3. noble gases
4. Lanthanides and Actinides
16
New cards
63\.546 g/mole
What is the molar mass of Cu (Copper)?
(picture of the periodic table included)
1. 35.4527 g/mole
2. 58.933200 g/mole
3. 29 g/mole
4. 63.546 g/mole
(picture of the periodic table included)
1. 35.4527 g/mole
2. 58.933200 g/mole
3. 29 g/mole
4. 63.546 g/mole
17
New cards
rust
Check the following that are chemical changes:
\
1. condense
2. crush
3. rust
4. melt
5. dissolve
\
1. condense
2. crush
3. rust
4. melt
5. dissolve
18
New cards
10 moles
How many moles are there in 584.43 grams of NaCl? The molar masses of the elements are the following: Na=22.990 g/mole, Cl=35.453 g/mole
\
1. 20 moles
2. 10 moles
3. 10.000039354739526 moles
4. 5 moles
\
1. 20 moles
2. 10 moles
3. 10.000039354739526 moles
4. 5 moles
19
New cards
The 3D region around the nucleus where the electron is found 90% of the time
Which choice best defines an atomic orbital?
\
1. The circular path an electron takes around the nucleus of an atom
2. The 3D region around the nucleus where the electron is found 90% of the time
3. The principle energy level of the electron
4. Aufbau's rule describes atomic orbitals
\
1. The circular path an electron takes around the nucleus of an atom
2. The 3D region around the nucleus where the electron is found 90% of the time
3. The principle energy level of the electron
4. Aufbau's rule describes atomic orbitals
20
New cards
All choices indicate a reaction has taken place
Which of the following indicates that a chemical reaction has taken place?
\
1. Gas bubbles are formed
2. Heat is produced
3. There is a color change
4. All choices indicate a reaction has taken place
\
1. Gas bubbles are formed
2. Heat is produced
3. There is a color change
4. All choices indicate a reaction has taken place
21
New cards
salt dissolved in water
Check the following that are homogeneous mixtures:
\
1. vegetable soup
2. salt dissolved in water
3. a mixture of oil and water
4. Water
5. Copper metal
\
1. vegetable soup
2. salt dissolved in water
3. a mixture of oil and water
4. Water
5. Copper metal
22
New cards
PERIODS !!!!!!
What are the horizontal rows on the periodic table called?
\
1. periods
2. noble gases
3. f block
4. groups
\
1. periods
2. noble gases
3. f block
4. groups
23
New cards
metals and nonmetals
What does the bright pink staircase line divide on the periodic table?
\
1. solids and gases
2. metals and nonmetals
3. electronegative and electropositive elements
4. d sublevel and p sublevel
\
1. solids and gases
2. metals and nonmetals
3. electronegative and electropositive elements
4. d sublevel and p sublevel
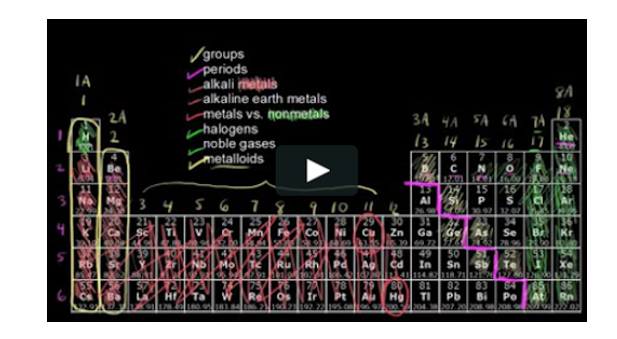
24
New cards
196\.97 amu
The average atomic mass of Gold, atomic number 79 is
\
1. 275.97 amu
2. not shown on the periodic table
3. 196.97 amu
4. 79 amu
\
1. 275.97 amu
2. not shown on the periodic table
3. 196.97 amu
4. 79 amu
25
New cards
a metal and a nonmetal
An ionic bond generally occurs between
\
1. a metal and a nonmetal
2. two metals
3. any two atoms
4. two nonmetals
\
1. a metal and a nonmetal
2. two metals
3. any two atoms
4. two nonmetals
26
New cards
neutrons, mass numbers
Isotopes of an element have different numbers of __________, which result in different ______________.
\
1. neutrons, atomic numbers
2. neutrons, protons
3. neutrons, mass numbers
4. protons, electrons
\
1. neutrons, atomic numbers
2. neutrons, protons
3. neutrons, mass numbers
4. protons, electrons
27
New cards
s block
Groups 1 and 2 and He make up the
\
1. p block
2. f block
3. s block
4. d block
\
1. p block
2. f block
3. s block
4. d block
28
New cards
b (1 covalent bond has 2 electrons shared, and it has 1 bond on each side total of 4 electrons/dots)
Which of the following molecules has two single covalent bonds?
\
1. a
2. b
3. c
4. d
5. e
6. f
\
1. a
2. b
3. c
4. d
5. e
6. f
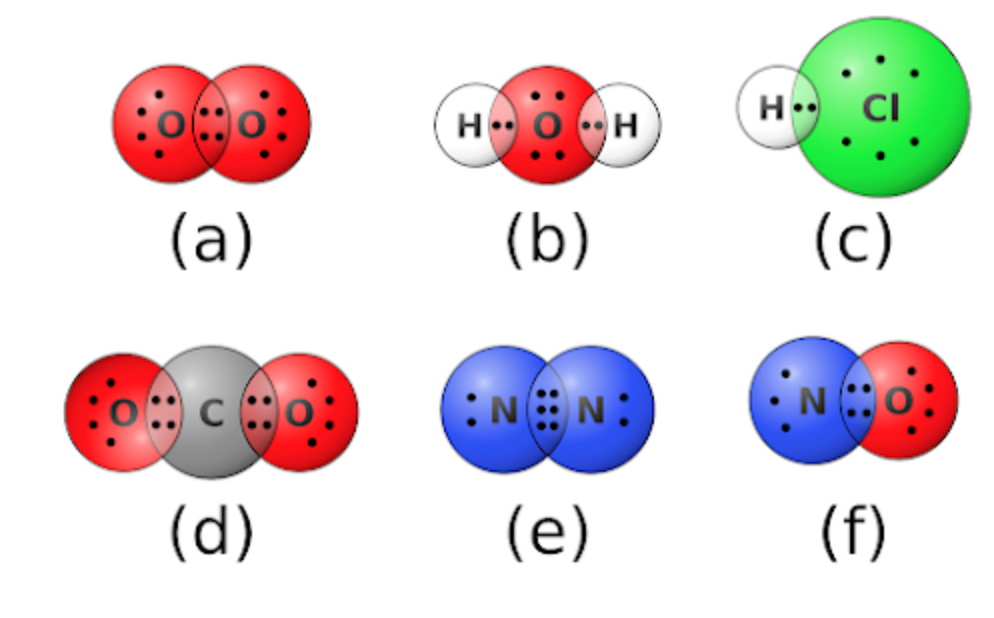
29
New cards
Lanthanides
Elements 57-71 are called the
\
1. Noble gases
2. Lanthanides
3. Actinides
4. f block
\
1. Noble gases
2. Lanthanides
3. Actinides
4. f block
30
New cards
Lewis Diagram
A diagram that shows the element symbol surrounded by dots representing valence electrons and lines that represent pairs of shared electrons is called a/an
\
1. Octet Rule
2. Lewis Diagram
3. Shell Diagram
4. Molecular Diagram
\
1. Octet Rule
2. Lewis Diagram
3. Shell Diagram
4. Molecular Diagram
31
New cards
Zirconium
An atom has 40 electrons. Which element is it?
\
1. Calcium
2. Argon
3. Potassium
4. Zirconium
\
1. Calcium
2. Argon
3. Potassium
4. Zirconium
32
New cards
two nonmetals
A covalent bond generally occurs between
1. two nonmetals
2. two metals
3. any two atoms
4. a metal and a nonmetal
1. two nonmetals
2. two metals
3. any two atoms
4. a metal and a nonmetal
33
New cards
The Law of Conservation of Mass
The Law that states that the mass of the reactants in a chemical reaction are equal to the mass of the products in the reaction is
\
1. The Law of Multiple Proportions
2. The Law of Conservation of Mass
3. The Law of Conservation of Energy
4. The Law of Definite Proportions
\
1. The Law of Multiple Proportions
2. The Law of Conservation of Mass
3. The Law of Conservation of Energy
4. The Law of Definite Proportions
34
New cards
become a vapor or gas
Adding heat to a liquid will eventually cause it to
\
1. become a solid
2. become a vapor or gas
3. dry up and disappear
4. catch on fire
\
1. become a solid
2. become a vapor or gas
3. dry up and disappear
4. catch on fire
35
New cards
a heterogeneous mixture
A sand and water mixture would be considered
\
1. a homogeneous mixture
2. a compound
3. a heterogeneous mixture
4. a pure substance
\
1. a homogeneous mixture
2. a compound
3. a heterogeneous mixture
4. a pure substance
36
New cards
Sodium
The following is the electron configuration for
\
1. Hydrogen
2. Lithium
3. Sodium
4. Neon
\
1. Hydrogen
2. Lithium
3. Sodium
4. Neon
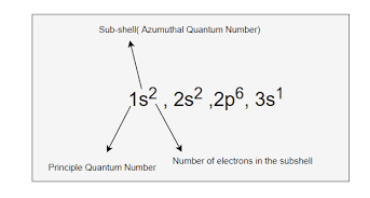
37
New cards
6
How many electrons are shared between two atoms when there is a triple covalent bond?
1. 6
2. 4
3. 3
4. 2
1. 6
2. 4
3. 3
4. 2
38
New cards
3
How many sodium atoms are in the compound below?
\
1. 5
2. 3
3. 6
4. 21
\
1. 5
2. 3
3. 6
4. 21
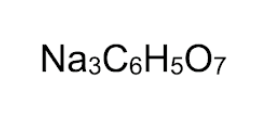
39
New cards
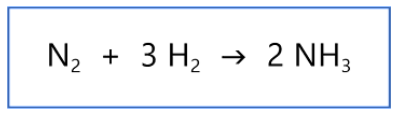
2 X 14.007 + 6 X 1.0079
Choose the correct operation to find the molar mass of the product of this chemical reaction. The molar mass of N is 14.007 g/mole and the molar mass of H is 1.0079 g/mole.
\
1. 2 X 14.007 + 6 X 1.0079
2. 6 X 14.007 + 6 X 1.0079
3. 2 X 14.007 + 3 X 1.0079
4. 2 X 14.007 X 6 X 1.0079
\
1. 2 X 14.007 + 6 X 1.0079
2. 6 X 14.007 + 6 X 1.0079
3. 2 X 14.007 + 3 X 1.0079
4. 2 X 14.007 X 6 X 1.0079
40
New cards
Lithium
Calculate the atomic mass of element X. 7.5% of the isotopes have a mass of 6.015 amu and 92.5% of the isotopes have a mass of 7.016. Which element is X?
\
1. Carbon
2. Boron
3. Nitrogen
4. Lithium
\
1. Carbon
2. Boron
3. Nitrogen
4. Lithium
41
New cards
principle energy level !!!!!!!!!!!!!!
What does the "1" stand for in this electron configuration?
\
1. electron
2. energy sublevel
3. orbital
4. principle energy level
\
1. electron
2. energy sublevel
3. orbital
4. principle energy level
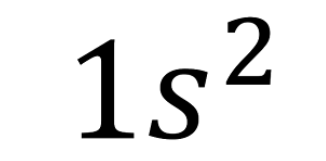
42
New cards
cobalt
What element does this orbital diagram represent?
\
1. Zinc
2. Iron
3. Cobalt
4. Nickel
\
1. Zinc
2. Iron
3. Cobalt
4. Nickel
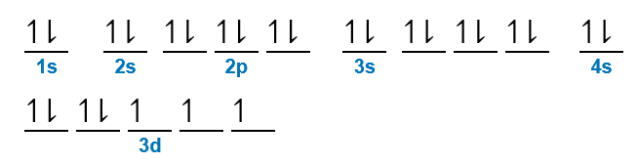
43
New cards
proton
The subatomic particle that gives an atom its identity is the
\
1. neutron
2. electron
3. proton
4. nucleus
\
1. neutron
2. electron
3. proton
4. nucleus
44
New cards
the mass number of this isotope of Kr
What does the number 84 represent?
\
1. the mass number of this isotope of Kr
2. the number of neutrons of this isotope of Kr
3. the number of protons of this isotope of Kr
4. the number of electrons of this isotope of Kr
\
1. the mass number of this isotope of Kr
2. the number of neutrons of this isotope of Kr
3. the number of protons of this isotope of Kr
4. the number of electrons of this isotope of Kr

45
New cards
the mass in grams of one mole of a substance
Molar mass is ______________.
\
1. equal to 602 sextillion grams
2. the mass in grams of one mole of a substance
3. what gets extracted when you have your wisdom teeth removed
4. not found on the periodic table
\
1. equal to 602 sextillion grams
2. the mass in grams of one mole of a substance
3. what gets extracted when you have your wisdom teeth removed
4. not found on the periodic table
46
New cards
5\.2 moles CH4 divided by the molar mass of methane
Which one of the following mathematical operations answers the question "How many grams are there in 5.2 moles of methane gas?"
\
1. 5.2 moles CH4 divided by the molar mass of methane
2. The molar mass of CH4 divided by 5.2 moles
3. The molar mass of CH4 divided by Avogadro's number
4. 5.2 moles CH4 multiplied by the molar mass of methane
\
1. 5.2 moles CH4 divided by the molar mass of methane
2. The molar mass of CH4 divided by 5.2 moles
3. The molar mass of CH4 divided by Avogadro's number
4. 5.2 moles CH4 multiplied by the molar mass of methane

47
New cards
the particles are far apart and move rapidly
Choose the following that describes the behavior of the particles that make up a gas:
\
1. the particles are loosely connected to each other
2. the particles are far apart and move rapidly
3. The particles move farther apart if the temperature decreases
4. the particles are close together and vibrate
\
1. the particles are loosely connected to each other
2. the particles are far apart and move rapidly
3. The particles move farther apart if the temperature decreases
4. the particles are close together and vibrate
48
New cards
Mgl2 (for magnesium iodine, MgO for magnesium oxide) (in the question it says magnesium oxide, but in the pic and answer choices it says magnesium iodine? tell cavallo for an extra point maybe)
What is the formula of Magnesium oxide?
(all numbers are subscripts)
1. Mg2l
2. Mg2l2
3. Mgl2
4. Mgl
(all numbers are subscripts)
1. Mg2l
2. Mg2l2
3. Mgl2
4. Mgl
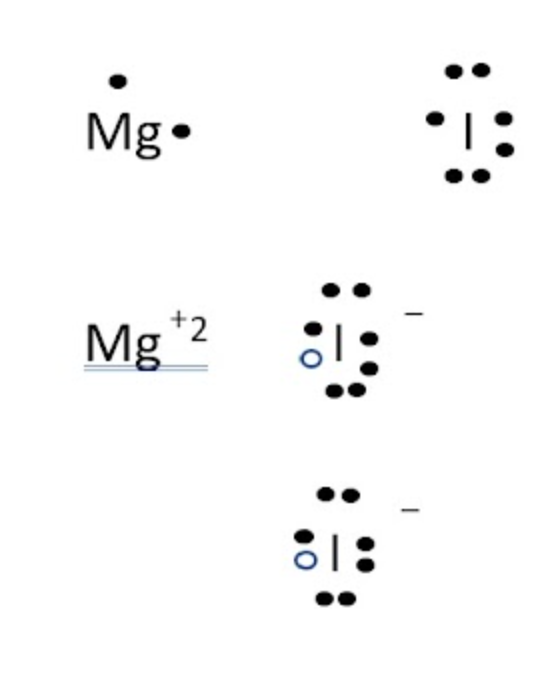
49
New cards
using a Periodic Table to look up the molar mass which is listed under the element name
You can find out how much a mole of sulfur weighs by
\
1. using a Periodic Table to look up the molar mass which is listed under the element name
2. asking your medical doctor
3. counting out all of the atoms and then weighing them on a scale
4. multiplying the molar mass of sulfur by Avogadro's number
\
1. using a Periodic Table to look up the molar mass which is listed under the element name
2. asking your medical doctor
3. counting out all of the atoms and then weighing them on a scale
4. multiplying the molar mass of sulfur by Avogadro's number
50
New cards
bonds between atoms in reactants are broken, bonds between atoms in products are formed.
During a chemical reaction,
\
1. atoms are created.
2. atoms are destroyed.
3. bonds between atoms in reactants are broken, bonds between atoms in products are formed.
4. bonds between atoms in products are broken, bonds between atoms in reactants are formed.
\
1. atoms are created.
2. atoms are destroyed.
3. bonds between atoms in reactants are broken, bonds between atoms in products are formed.
4. bonds between atoms in products are broken, bonds between atoms in reactants are formed.
51
New cards
Law of conservation of mass
Balanced chemical equations show that the _________ is being followed in a chemical reaction.
\
1. Law of conservation of mass
2. Law of definite proportions
3. Law of balanced equations
4. Law of cause and effect
\
1. Law of conservation of mass
2. Law of definite proportions
3. Law of balanced equations
4. Law of cause and effect
52
New cards
2, 3, 2, 2
Balance the following equation. The correct coefficients (in order) are:
\
1. 2, 3, 2, 2
2. 3, 3, 1, 1
3. 6, 6, 3, 1
\
1. 2, 3, 2, 2
2. 3, 3, 1, 1
3. 6, 6, 3, 1
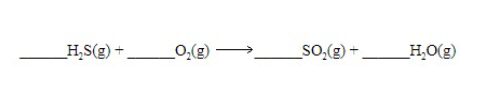
53
New cards
endothermic (exo is negative, and since the difference is positive meaning the temperature increased after the reaction, it’s endo/positive)
The energy calculated to break bonds in the reactants of a chemical reaction is 2500 kJ. The energy released when bonds form in the products is 3000 kJ. This reaction is
1. endothermic
2. exothermic
3. combustion
4. nonspontaneous
1. endothermic
2. exothermic
3. combustion
4. nonspontaneous
54
New cards
valence electrons
The highest energy level, outermost electrons in an atom that are involved in chemical bonds are called ____________________ .
\
1. shell electrons
2. outside electrons
3. vital electrons
4. valence electrons
\
1. shell electrons
2. outside electrons
3. vital electrons
4. valence electrons
55
New cards
yields or produces
An arrow in an chemical equation can be read as
\
1. yields or produces
2. replaces
3. consumes
4. equals
\
1. yields or produces
2. replaces
3. consumes
4. equals
56
New cards
1
How many orbitals does an s sublevel have?
\
1. 1
2. 3
3. 2
4. 5
\
1. 1
2. 3
3. 2
4. 5
57
New cards
3
How many orbitals does a p sublevel have?
\
1. 1
2. 3
3. 2
4. 5
\
1. 1
2. 3
3. 2
4. 5
58
New cards
5
How many orbitals does a d sublevel have?
\
1. 1
2. 3
3. 2
4. 5
\
1. 1
2. 3
3. 2
4. 5
59
New cards
7
How many orbitals does an f sublevel have?
\
1. 1
2. 3
3. 2
4. 5
5. 7
\
1. 1
2. 3
3. 2
4. 5
5. 7
60
New cards
True
It is important that when you write an element symbol, the first letter is capitalized and the second letter is lowercase.
\
1. True
2. False
\
1. True
2. False
61
New cards
A diagram of the valence electrons in an atom.
What is an electron dot diagram?
\
1. A diagram of the core electrons in an atom.
2. A diagram of the protons in an element.
3. A diagram of the valence electrons in an atom.
4. A diagram of all electrons in an atom.
\
1. A diagram of the core electrons in an atom.
2. A diagram of the protons in an element.
3. A diagram of the valence electrons in an atom.
4. A diagram of all electrons in an atom.
62
New cards
6\.02 x 10^23
How many jelly beans are in a mole of jelly beans?
1. 602,000
2. a gazillion
3. 6.02 x 10^23
4. a few dozen
1. 602,000
2. a gazillion
3. 6.02 x 10^23
4. a few dozen
63
New cards
substances and mixtures
All matter can be divided into
\
1. homogeneous or heterogeneous mixtures
2. substances and mixtures
3. problems and solutions
4. elements and compounds
\
1. homogeneous or heterogeneous mixtures
2. substances and mixtures
3. problems and solutions
4. elements and compounds
64
New cards
The Quantum Mechanical model
What is the current model of the atom called?
\
1. The Quantum Mechanical model
2. The Plumb-pudding model
3. The Bohr model
4. The Planetary model
\
1. The Quantum Mechanical model
2. The Plumb-pudding model
3. The Bohr model
4. The Planetary model
65
New cards
compound
A pure substance made of two different elements bonded together is called a
\
1. homogeneous mixture
2. compound
3. product
4. heterogeneous mixture
\
1. homogeneous mixture
2. compound
3. product
4. heterogeneous mixture
66
New cards
covalent
A bond between two nonmetals that have a large difference in their electronegativity values would be best classified as
1. polar covalent
2. ionic
3. covalent
4. nonpolar covalent
1. polar covalent
2. ionic
3. covalent
4. nonpolar covalent
67
New cards
ion
An atom that loses or gains electrons and has a charge is called a/an____________.
\
1. compound
2. isotope
3. mixture
4. ion
\
1. compound
2. isotope
3. mixture
4. ion
68
New cards
waves
The quantum mechanical model of the atom takes into account that electrons behave as both particles and
\
1. waves
2. gamma rays
3. neutrons
4. protons
\
1. waves
2. gamma rays
3. neutrons
4. protons
69
New cards
reactants
In a chemical equation, the elements and compounds to the left of the arrow are called the
\
1. reactants
2. products
3. coefficients
4. yields
\
1. reactants
2. products
3. coefficients
4. yields
70
New cards
Nitrogen
The atoms below could be
\
1. Hydrogen
2. Carbon
3. Fluorine
4. Nitrogen
\
1. Hydrogen
2. Carbon
3. Fluorine
4. Nitrogen
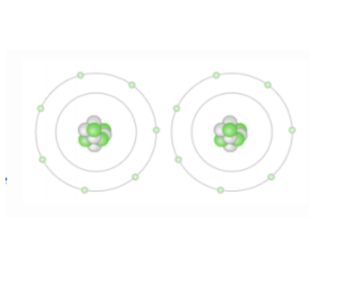
71
New cards
6
An atom has a mass number of 14 and an atomic number of 6. How many electrons does it have?
\
1. 8
2. 6
3. 20
4. 14
\
1. 8
2. 6
3. 20
4. 14
72
New cards
a metal and an nonmetal ion
An ionic bond forms between
\
1. two metal ions
2. two nonmetal ions
3. a metal and an nonmetal ion
4. any two ions
\
1. two metal ions
2. two nonmetal ions
3. a metal and an nonmetal ion
4. any two ions
73
New cards
pic is the answer
Which is the correct dot diagram for the element carbon?
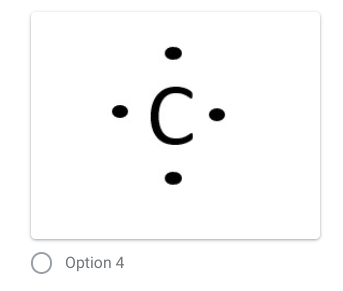
74
New cards
Carbon-14
The proper way to indicate the isotope of carbon (atomic number 6) that has 8 neutrons is:
\
1. Carbon-6
2. Carbon-16
3. Carbon-12
4. Carbon-14
\
1. Carbon-6
2. Carbon-16
3. Carbon-12
4. Carbon-14
75
New cards
noble gases
What are the elements in the last group (8A or 18) on the periodic table called?
\
1. actinides
2. noble gases
3. p block
4. lanthanides
\
1. actinides
2. noble gases
3. p block
4. lanthanides
76
New cards
48
How many neutrons does this isotope of Kr have?
\
1. 84
2. 36
3. 120
4. 48
\
1. 84
2. 36
3. 120
4. 48
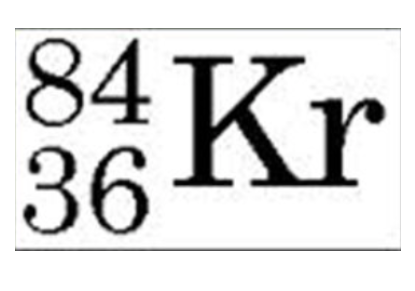
77
New cards
cation
An atom that loses electrons becomes a(n)...
\
1. anion
2. cation
3. positron
4. Higgs Boson
\
1. anion
2. cation
3. positron
4. Higgs Boson
78
New cards
First, look up the molar mass of a hydrogen atom, then multiply it times 2. Then look up the molar mass of an oxygen atom and add it to the number you got in the first step.
How would you figure out the molar mass of a water molecule?
\
1. First, look up the molar mass of a hydrogen atom, then multiply it times 2. Then look up the molar mass of an oxygen atom and add it to the number you got in the first step.
2. First, look up "water" on the Periodic Table, then add Avogadro's Number to it.
3. First, add the number of hydrogen molecules to the number of oxygen molecules. Then make sure you have a balanced equation.
4. Divide the number of moles by the number of particles in one mole.
\
1. First, look up the molar mass of a hydrogen atom, then multiply it times 2. Then look up the molar mass of an oxygen atom and add it to the number you got in the first step.
2. First, look up "water" on the Periodic Table, then add Avogadro's Number to it.
3. First, add the number of hydrogen molecules to the number of oxygen molecules. Then make sure you have a balanced equation.
4. Divide the number of moles by the number of particles in one mole.

79
New cards
Liquid
Which state of matter has a definite volume, but no definite shape?
\
1. Gas
2. Plasma
3. Solid
4. Liquid
\
1. Gas
2. Plasma
3. Solid
4. Liquid
80
New cards
ionic
What type of bond will form between Lithium (Li) and Chlorine (Cl)?
\
1. metallic
2. polar covalent
3. ionic
4. covalent
\
1. metallic
2. polar covalent
3. ionic
4. covalent
81
New cards
False
The period in which an element occurs, will tell you how many valence electrons an atom has.
\
1. True
2. False
\
1. True
2. False
82
New cards
physical
Chlorine gas is yellowish in color. This is an example of a __________ property of chlorine.
\
1. physical
2. heterogeneous
3. homogeneous
4. chemical
\
1. physical
2. heterogeneous
3. homogeneous
4. chemical
83
New cards
molecule
A group of two or more nonmetal atoms joined by covalent bonds is called a
1. compound
2. formula unit
3. molecule
4. chemical
1. compound
2. formula unit
3. molecule
4. chemical
84
New cards
6
How many total Hydrogen atoms are there in the product of the balanced chemical equation?
\
1. 2
2. 3
3. 6
4. 4
\
1. 2
2. 3
3. 6
4. 4
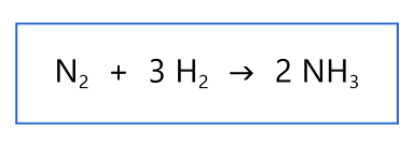
85
New cards
endothermic (absorbs heat, so it feels cold; exothermic releases heat, so it feels hot/warm)
Which type of chemical reaction will feel cold to the touch?
1. exothermic
2. covalent
3. ionic
4. endothermic
1. exothermic
2. covalent
3. ionic
4. endothermic
86
New cards
both the mass and abundance of the atom's isotopes
The average atomic mass of an atom is usually not a whole number because it takes into account
\
1. the mass of each of the atom's isotopes
2. the abundance of the atom's isotopes in nature
3. both the mass and abundance of the atom's isotopes
4. the mass of the protons and electrons of the atom
\
1. the mass of each of the atom's isotopes
2. the abundance of the atom's isotopes in nature
3. both the mass and abundance of the atom's isotopes
4. the mass of the protons and electrons of the atom
87
New cards
nucleus of the atom
Earnest Rutherford used the gold foil experiment to discover the
\
1. Plumb pudding model of the atom
2. Quantum mechanical model of the atom
3. nucleus of the atom
4. Planetary model of the atom
\
1. Plumb pudding model of the atom
2. Quantum mechanical model of the atom
3. nucleus of the atom
4. Planetary model of the atom
88
New cards
protons and electrons
An atom is neutral because it has the same number of ___________ and ____________.
\
1. protons, neutrons and electrons
2. neutrons and electrons
3. protons and electrons
4. protons and neutrons
\
1. protons, neutrons and electrons
2. neutrons and electrons
3. protons and electrons
4. protons and neutrons
89
New cards
a homogeneous mixture
In chemistry, a solution is
\
1. a mixture of two metals
2. a pure substance
3. a heterogeneous mixture
4. a homogeneous mixture
\
1. a mixture of two metals
2. a pure substance
3. a heterogeneous mixture
4. a homogeneous mixture
90
New cards
electron
The part of the atom that is important in creating bonds with other atoms is the
\
1. proton
2. electron
3. neutron
4. nucleus
\
1. proton
2. electron
3. neutron
4. nucleus
91
New cards
synthesis
The following equation is a _______________ reaction
1. decomposition
2. combustion
3. synthesis
4. double replacement
5. single replacement
1. decomposition
2. combustion
3. synthesis
4. double replacement
5. single replacement
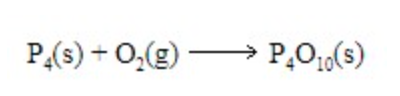
92
New cards
6 electrons
How many electrons will a p sublevel hold?
\
1. 6 electrons
2. 3 electrons
3. 2 electrons
4. 10 electrons
\
1. 6 electrons
2. 3 electrons
3. 2 electrons
4. 10 electrons
93
New cards
Chromium
The mass number of an atom is 52 and the atomic number is 24. Which element is this?
\
1. Osmium
2. Nickel
3. Tellurium
4. Chromium
\
1. Osmium
2. Nickel
3. Tellurium
4. Chromium
94
New cards
3
The molar mass of carbon is 12.011 g. How many moles do you have if you have 36.033 g of carbon? Please select the answer closest to what appears in your calculator, do not round.
\
1. 602 sextillion moles
2. 3.000074933184564 moles
3. 432.792363 moles
4. 3 moles
\
1. 602 sextillion moles
2. 3.000074933184564 moles
3. 432.792363 moles
4. 3 moles
95
New cards
Potassium transferred it's one valence electron to Chlorine, ions formed and an ionic bond resulted.
The top diagram shows you a neutral potassium atom and a neutral chlorine atom. What does the bottom diagram indicate?
\
1. Potassium transferred all 8 of its electrons to chlorine.
2. Potassium gained an electron while chlorine lost an electron.
3. Potassium forms an anion and chlorine forms a cation to form an ionic bond.
4. Potassium transferred it's one valence electron to Chlorine, ions formed and an ionic bond resulted.
\
1. Potassium transferred all 8 of its electrons to chlorine.
2. Potassium gained an electron while chlorine lost an electron.
3. Potassium forms an anion and chlorine forms a cation to form an ionic bond.
4. Potassium transferred it's one valence electron to Chlorine, ions formed and an ionic bond resulted.
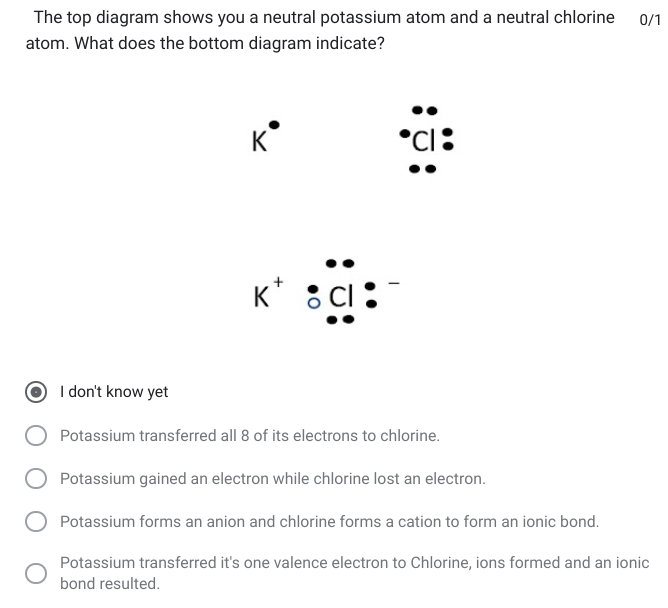
96
New cards
electronegativity value
The values on the periodic table below represent the element's
1. atmic number
2. ionization energy value
3. electronegativity value
4. atomic mass
1. atmic number
2. ionization energy value
3. electronegativity value
4. atomic mass
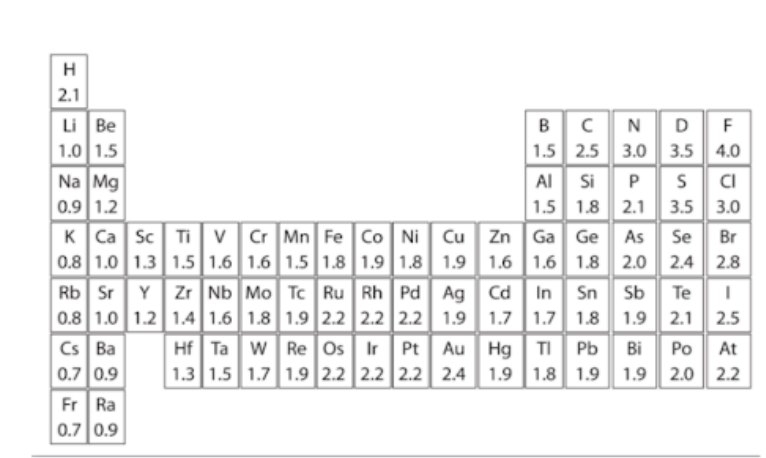
97
New cards
coefficients, subscripts
When balancing equations, you can add _________ but you can never add or change _________.
\
1. atoms, compounds
2. superscripts, subscripts
3. subscripts, coefficients
4. coefficients, subscripts
\
1. atoms, compounds
2. superscripts, subscripts
3. subscripts, coefficients
4. coefficients, subscripts
98
New cards
4
Carbon has _____________ valence electrons.
\
1. 4
2. 0
3. 14
4. 6
\
1. 4
2. 0
3. 14
4. 6