Microbiology Chapter 2: Molecules of Life
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
Chemistry
study of atoms and molecules
molecules
2 or more atoms joined together
compound
2 or more different atoms joined together
ionic
transfer e-, positive and negative joined together
covalent
sharing e-
chemical reaction
reactants → products
ex. 2H2 + O2 → 2H2O
Solvent
dissolves other things
solute
being dissolved
hydrophobic
repels water
hydrophilic
attracted to water, soluble
acid
release H+, 7-
base
accepts H+, 7+
Salt
acid + base → salt
doesn’t have O or OH, ionic, electrolytes
pH
logarithmic measure of the amount of H+
buffer
compound, maintains a stable pH
alcohol
R - OH
aldehyde
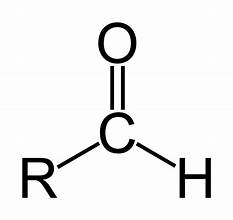
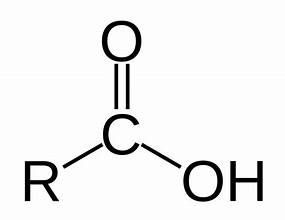
acid
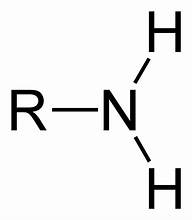
amine
ether
C - O - C
ester
C - (C = O) - OC
Ketone
C - (O = C) - C
4 Types of Organic Molecules
carbs, lipids, proteins, nucleic acids.
Carbohydrates
sugar whose function is energy
monosaccachrides
single unit sugars; glucose, fructose, galatose
disaccacharides
two unit sugars; sucrose, lactose, maltose
polysaccacharides
multiunit sugars; starch, cellulose, glycogens
lipids
fats and oils whose function is energy, membranes, and hormones
fatty acids
long chain of C and H with acid group one end
saturated
single bonds between carbons, molecule is straight
unsaturated
double bonds between carbons, bend, liquid
triglycerides
glycerol and 3 fatty acids attached
phospholipid
triglyceride with phosphate attached in place of one fatty acid
steroids
multiple ring lipids
proteins
amino acids, various structures(long chain, coil, flat sheet, folded)
function is enzymes, fibers, hormones, membranes, and antibodies
enzymes
proteins that cause reactions to occur or speed up
substrate
compound enzyme acts upon
active site
spot where substrate fits
denaturation
disruption of protein’s 3D shape
nucleic acids
general structure is base (ring with N)- sugar- one or more phosphates
adenosine phosphates
messengers and energy molecules
DNA and RNA
heredity and protein synthesis
FAD and NAD
metabolism
dehydration synthesis
joining of molecules
hydrolysis
breaking of molecules with water