Biology 1.1 and 1.2 Carbohydrates, monomers and polymers.
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
What is a monomer?
Monomer - a smaller repeating unit from which larger molecules called polymers are made.
Give some examples of monomers.
. Amino acids
. Nucleotides
. Monosaccharides
What is a polymer?
Polymer - large molecules made up of a large number of smaller repeating units called monomers joined together.
Polymers are formed in condensation reactions.
Define condensation.
A reaction that joins 2 molecules/monomers together by forming a chemical bond by removing a water molecule/releasing water.
Rule: remove one molecule of water for every two monomers.
Define hydrolysis.
Hydrolysis: breaking a chemical bond between 2 molecules/monomers by adding a water molecule/adding water.
e.g starch can be hydrolysed into 2 molecules of glucose.
Describe carbohydrates.
carbohydrates contain: carbon, hydrogen and water.
The general formula is CnH2nO
(This formula differs for disaccharides and trisaccharides because water has been removed from them).
Made up of the monomer 'monosacharide' which are single sugars.
What is a monosaccharide?
Monosaccharides are the monomers from which larger carbohydrates are made.
Monosaccharides are "single sugars".
Name the monosaccharides
glucose, fructose, galactose
What do all the monosaccharides have in common?
They are all hexoses with the formula C6H12O6
Pentoses and trioses can also be monosaccharides (the ones used in A level are only hexoses).
Describe how monosaccharides make up other molecules.
ALL monosaccharides make up disaccharides.
BUT ONLY glucose makes up the polysaccharides in the A level course.
describe disaccharides
Disaccharides are formed from the condensation reaction between 2 monosaccharides, forming a glycosidic bond.
KEY POINT: Disaccharides are held together by glycosidic bonds.
Name the disaccharides.
sucrose, lactose, maltose
Name the monosaccharides that make up each disaccharide.
Sucrose - fructose and glucose.
Maltose - two glucose molecules.
Lactose - glucose and galactose.
Name the 3 types of carbohydrate.
1. Monosaccharide
2. Disaccharide
3. Polysaccharide.
Describe Glucose.
Glucose is a hexose sugar, a monosaccharide, with the formula C6H12O6.
Glucose has two isomers: alpha and beta.
Glucose is the monomer/monosaccharide of polysaccharides.
Describe Beta glucose.
Beta-glucose is ring shaped and the OH at the end is in the upward position.
The hydroxyl groups are inverted.
Beta glucose is the monomer/monosaccharide of cellulose.
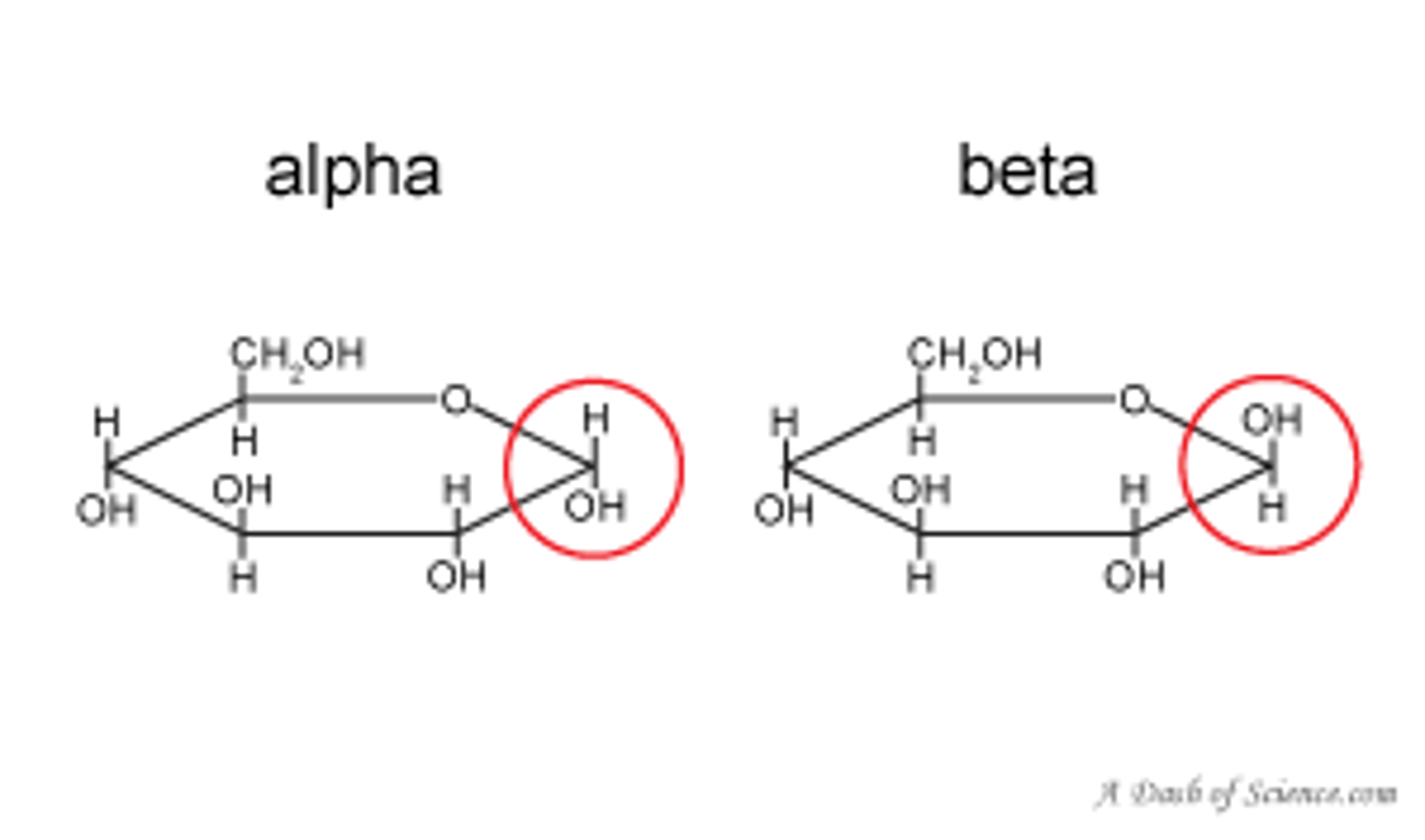
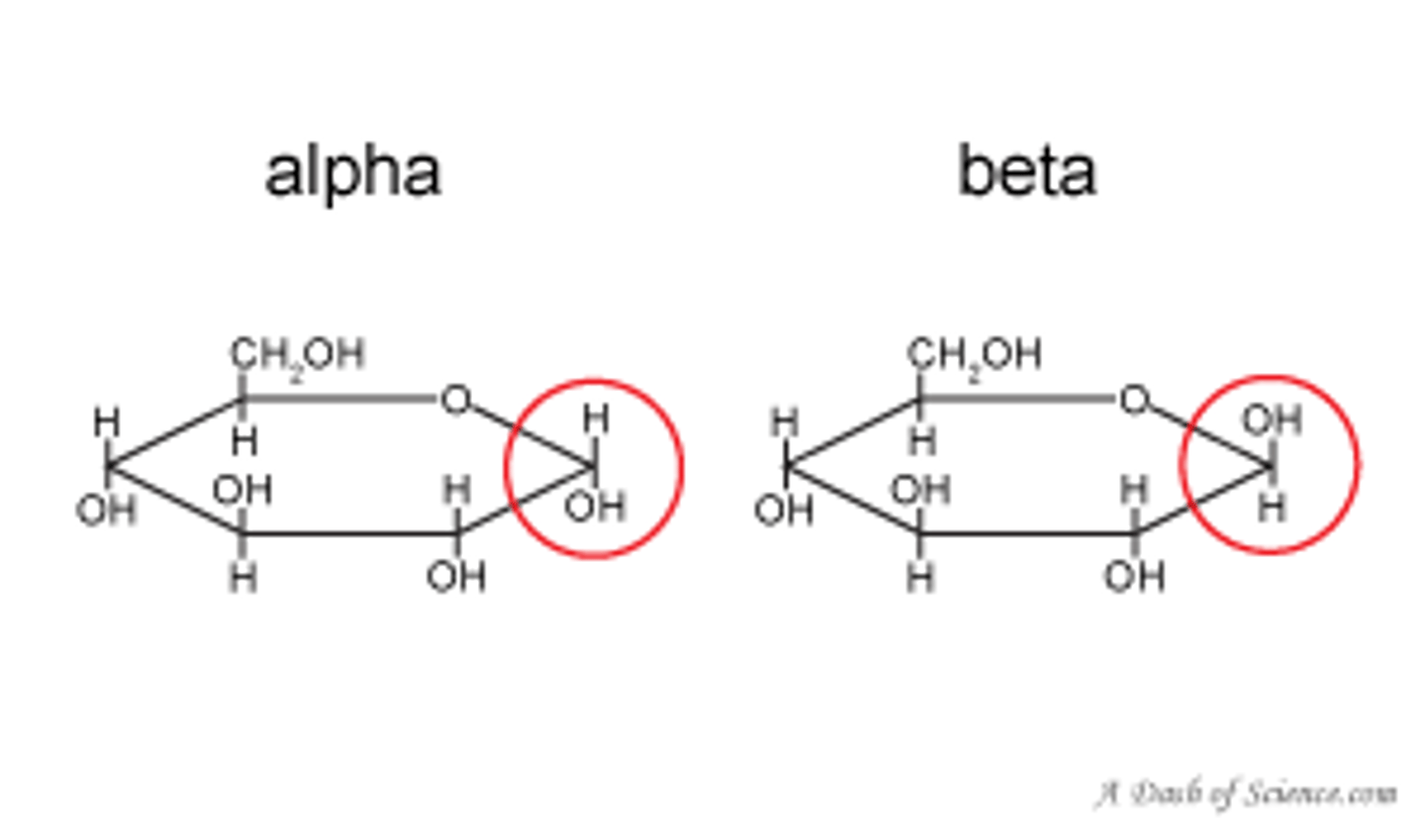
Describe alpha glucose
Alpha-glucose is ring shaped and the OH at the end is in the downward position.
. Hydroxyl groups are opposite eachother.
How are polysaccharides formed?
By the condensation of many glucose units
Describe some properties of polysaccharides.
Polysaccharides are insoluble; whereas monosaccharides and disaccharides are soluble.
Being insoluble means they do not affect the water potential of the cell.
Describe some properties of starch.
Starch is insoluble in water; does not affect the water potential of the cell.
Starch is a large molecule that can't leave the cell membrane.

Name the group in glucose.
Glucose has 2 hydroxyl groups (OH).
Name the polysaccharides and their monosaccharides.
Amylopectin - alpha glucose.
Amylose - alpha glucose.
Glycogen - alpha glucose.
Cellulose - Beta glucose.
Describe the functions of amylose and amylopectin in plants.
Starch is a store of alpha glucose in plants, it exists in two forms:
Amylose - long term store of glucose in plants.
For the purpose of overwintering and seeds.
Amylopectin - short term store of glucose.
For nightime when its dark and photosynthesis can't occur.
--> Plants need glucose for respiration
Describe the functions of glycogen in animal cells.
Glycogen is a glucose store in animals.
A dietary source of blood glucose
Also used in muscles to provide energy for muscle contraction.
Used in the liver for blood glucose regulation.
Describe the role of cellulose in plant cells.
Cellulose makes up plant cell walls.
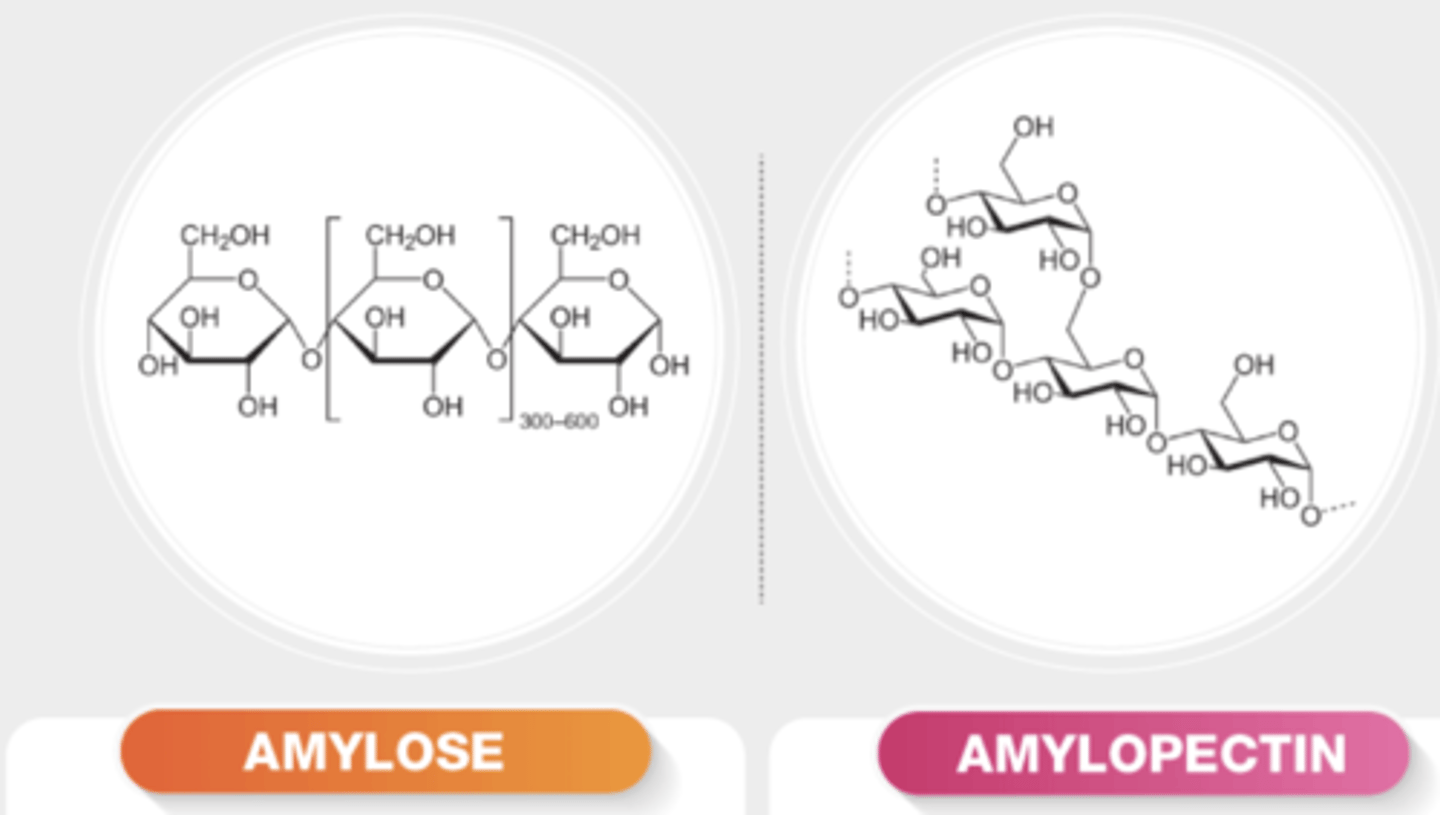
Describe the structure of Amylose.
Amylose is a polymer of Alpha glucose.
Amylose has glycosidic bonds that bring glucose units together at a slight angle so that they form a compact structure.
This helical shape means more starch units can be stored because glucose units are more compressed.
helical = more compact.
Describe the structure of Amylopectin.
Amylopectin is a polymer of alpha glucose.
Has 1,4 and 1,6 glycosidic bonds and is a very branched molecule. The multiple ends make it easy to hydrolyse into glucose for respiration in plants.
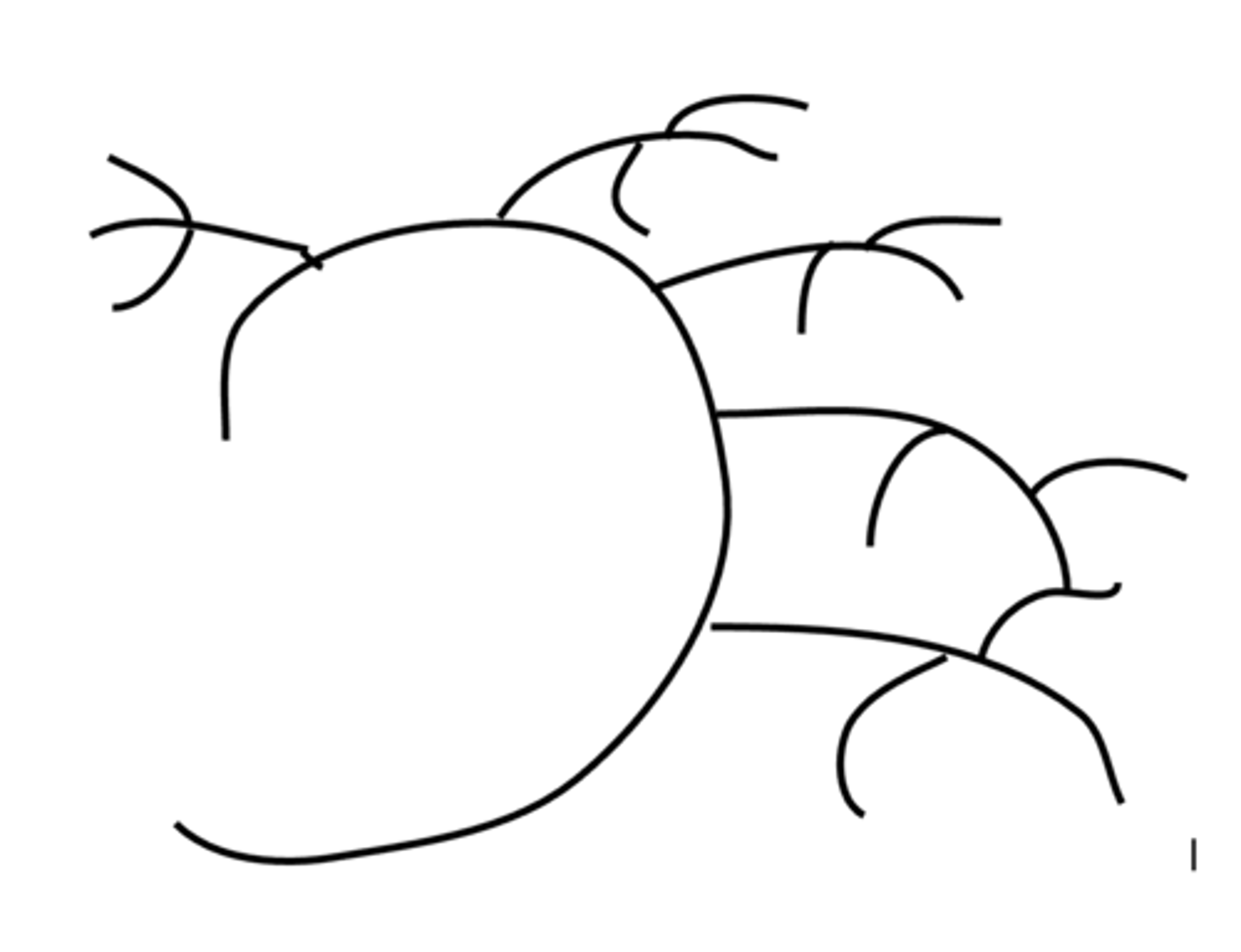
Describe the structure of Glycogen.
Glycogen is a polymer of alpha glucose.
A glucose store in animals.
Stored in the muscles and the liver.
Is a coiled molecule.
1,4 and 1,6 glycosidic bonds. Highly branched and easily hydrolysed into glucose for purposes such as blood glucose regulation and muscle contraction.
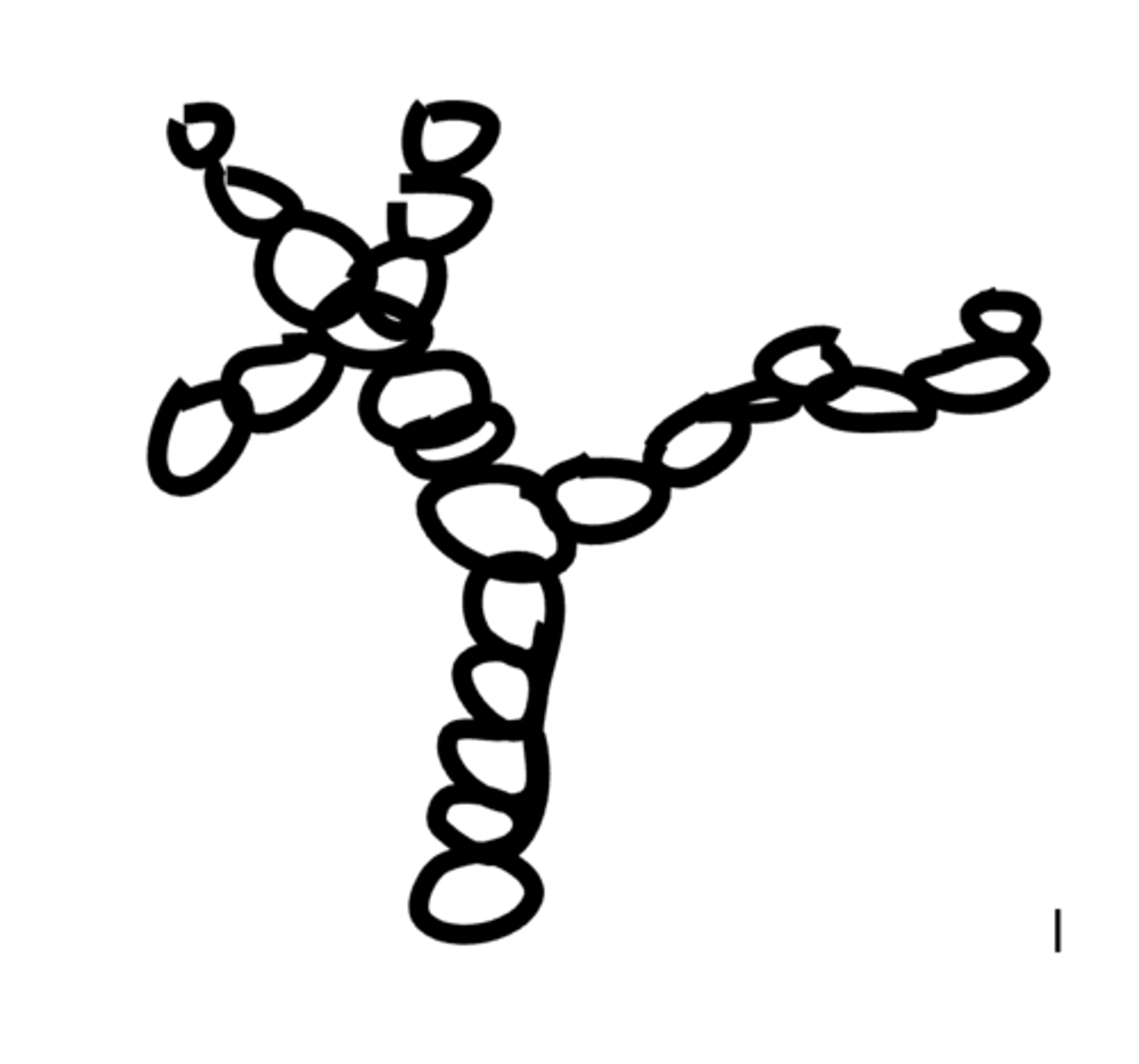
Describe the structure of Cellulose.
Polymer of Beta glucose.
Alternating B glucose units are 180 degrees to their neighbour so they form 1,4 glycosidic bonds.
Chains of beta glucose run parallel to each other and are held together by many hydrogen bonds.
Up to 2000 cellulose chains can form a thicker and stronger fibre called a microfibril.
Cellulose is a straight chain molecule, it has long and straight chains.
Describe how the structure of cellulose relates to its function in plant cells.
- Long and straight chains which can become linked together by many hydrogen bonds to form microfibrils which provide strength to the plant cell wall.
Describe how the structure of glycogen relates to its function in animal cells.
Glycogen is coiled and highly branched, can be easily hydrolysed into glucose for blood glucose regulation.
Describe the Biochemical test for reducing sugars.
1. Heat with Benedict's solution
2. Should observe a colour change on the colour range of
Blue ---> brick red precipitate.
Name some reducing sugars.
Reducing sugars are all disaccharides apart from Sucrose.
(e.g lactose, maltose).
Describe the colour scale for the colour change in the Benedicts test.
blue --> green ---> yellow ---> orange ---> brick red.
What does the colour change indicate?
The concentration of sugar.
Name a non-reducing sugar
sucrose, a disaccharide
Describe the Biochemical test for non-reducing sugars.
1. Heat with a dilute acid (the textbook names HCl)
--> heating with a dilute acid hydrolyses the non-reducing sugar into its monosaccharides.
2. Neutralise with a dilute alkali (the textbook names sodium hydrogencarbonate).
3. Heat with Benedict's solution.
4. blue ---> brick red precipitate.
What do we refer to Benedicts as in the exam?
A solution NOT reagent.
Give some limitations of the Benedicts test.
1. Semi quantitative
--> does not give the exact concentration of sugar.
2. Colour change is subjective.
3. Can't identify which sugars are present.
4. Can't be used with blood.
Describe the Biochemical test for starch.
1. Iodine/potassium iodide (KI preferred)
2. orange-brown ---> blue/black
Describe some methods we can use to determine the concentration of reducing sugar present in the Benedicts test.
Method 1:
. Filter the solution, dry the precipitate and weigh the precipitate to find out mass.
Method 2:
. Remove the precipitate by filtering and using a colorimeter to measure the absorbance of Benedict's solution.
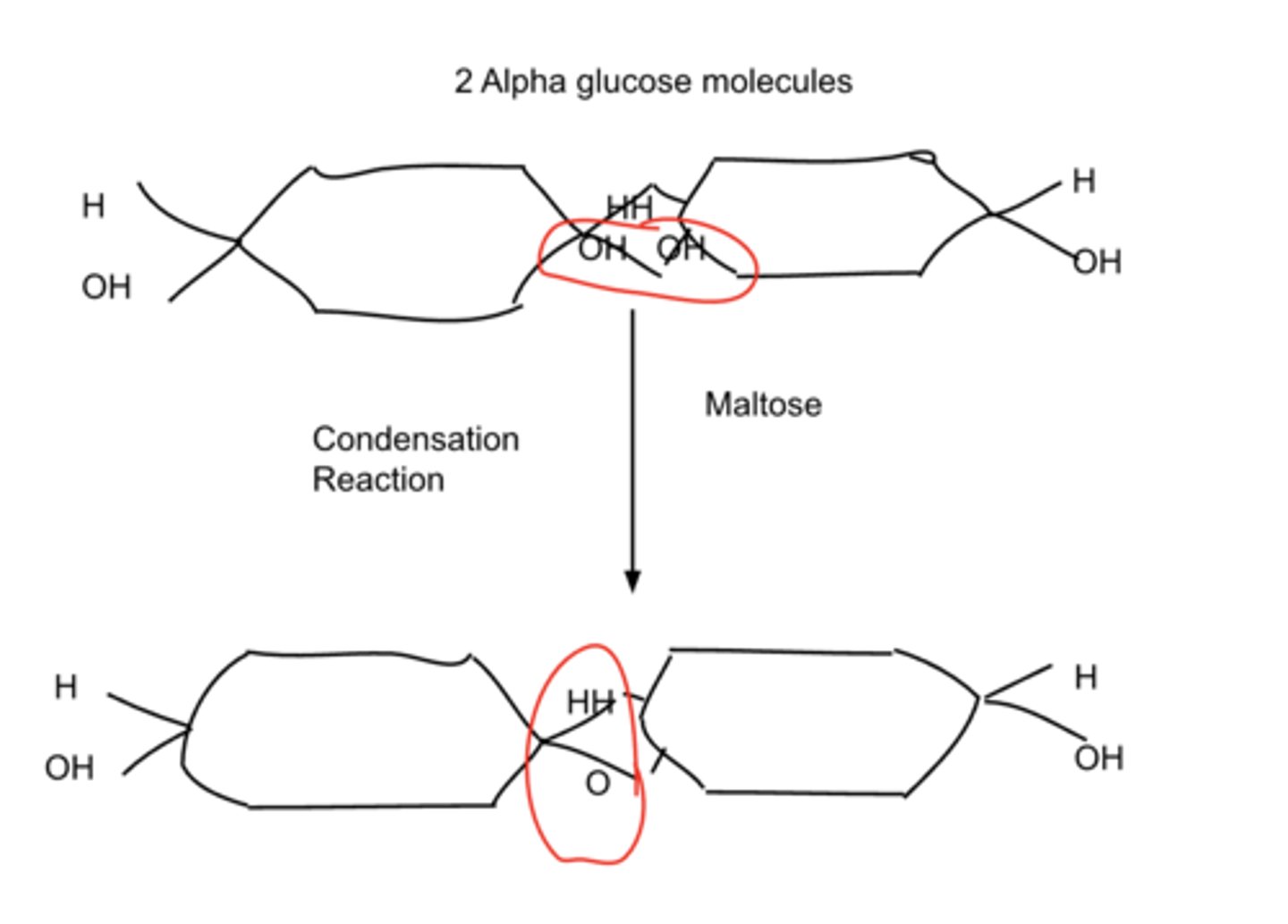
Draw a diagram to show the formation of the disaccharide maltose.
Condensation reaction between 2 alpha glucose molecules.
1 molecule of water removed.
Forms the disaccharide Maltose.
Removing the water forms a 1,4 glycosidic bond.
This reaction could be hydrolysed further to form Starch which is a 2 step hydrolysis reaction.
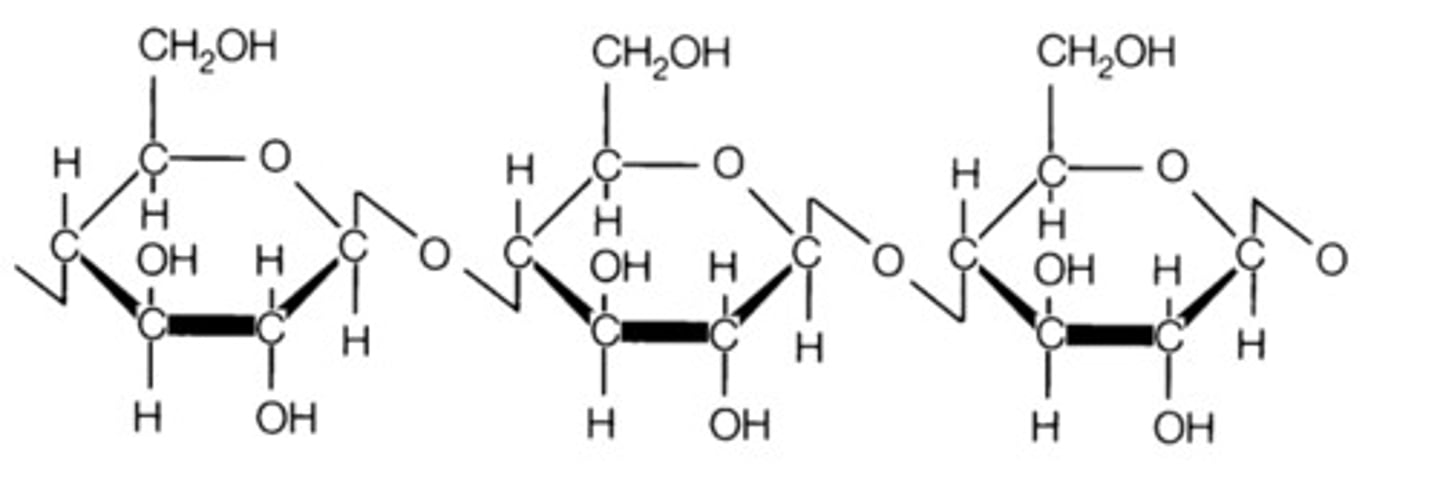
What is starch?
Starch: a stored form of glucose in plants
i.e amylose and amylopectin