Atomic Theory 2
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
What is the principle quantum number(n)?
Number of period or row of the periodic table
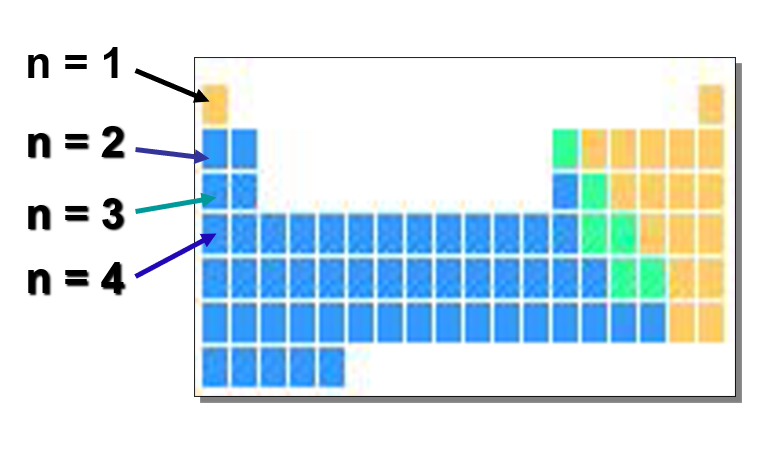
2
New cards
What is the angular quantum number(l)?
(n - 1) e.g.
if n=2
l=1
3
New cards
What is the magnetic quantum number(ml)?
if l=1
ml = -1,0,1
4
New cards
What is ms quantum number?
either -1/2 or +1/2 doesn’t matter