Oxidation and Reduction
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Oxidation
a compound or partial loss of electrons
Na(s) —> Na+ + 1e- sodium is oxidized
Fe(s) —> Fe2+ + 2e- is a half reaction
Iron lose electrons and oxidation becomes positive
Reduction
a complete or partial gain of election
O2 + 4e- —> 2O2- reduction - gains electrons becomes negative
oxygen is reduced
This is reaction 1. Fe(s) —> Fe2+ + 2e-
This is reaction 2. O2 + 4e- —> 2O2-
2(1) +2
2(Fe(s) —> Fe2+ + 2e- -)
+ O2 + 4e- —> 2O2- =
2Fe(s)+O2(g)+4e—>2Fe2++4e-+2O2-
2Fe(s)+O2(g)—>2FeO(s) Oxygen is reduced while Iron is oxidized. This is also a redox reaction.
Redox reaction
gaining and losing electrons from each other
LEO the lion goes GER
Lose electron oxidation
gain electron reduced
Oxidation numbers
A positive or negative number assigned to an atom to indicate its degree of oxidation or reduction
Monatomic ions have oxidation numbers equal to their charge
Na+ has a 1+
Fe3+ has a 3+
O2- has a -2
The oxidation number for hydrogen in a compound is +1 except in metal hydrides when it is -1. It’s like a 2 in 1. A wombo combo. A dual element.
+1 -1
LiH
Lithium lose e-
+1 -2
H2O
Oxygen steals H’s electron
The oxidation number for oxygen in a compound is -2 except for peroxides, where it is -1, or if it is positive with fluorine
No example but one from chat gpt
Hydrogen peroxide
+1 -1
H2O2
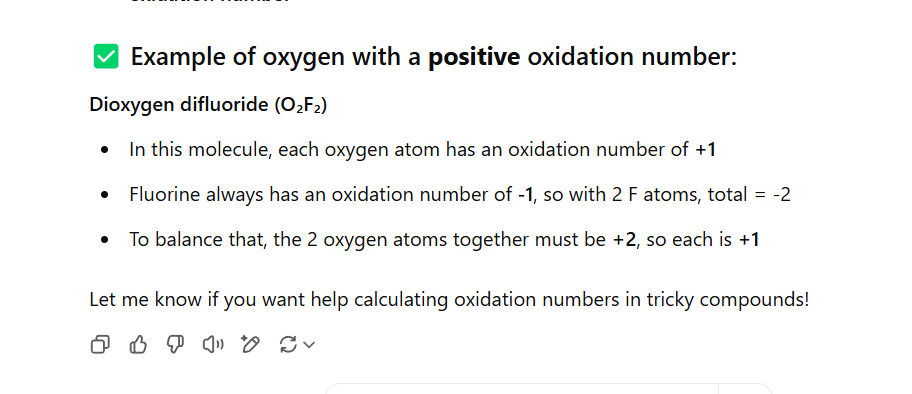
The oxidation number of any atom is elemental form is 0.
0 0 0
O2 H2 Fe
A neutral compound should have oxidation numbers that add to zero
Yet another non example but example from your good friend Chat

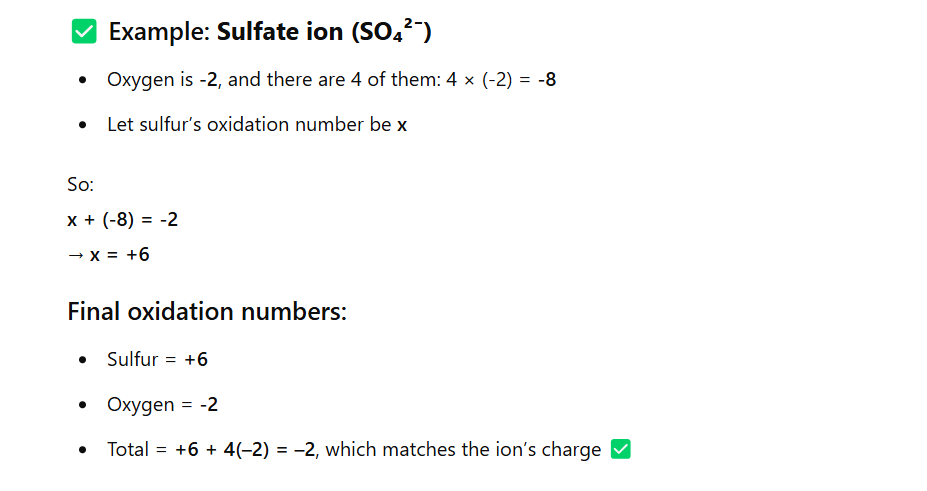
A polyatomic ion should have an oxidation number that adds to the overall charge
…