Giant covalent structures
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Each carbon atom is bonded to 4 other carbon atoms in which giant covalent structure?
diamond
Covalent bonds take a lot of energy to break so giant covalent structures have a...
a high melting point
The substance made of silicon and oxygen is...
silicon dioxide
A single sheet of carbon atoms where each bonded to three other carbon atoms
Graphene
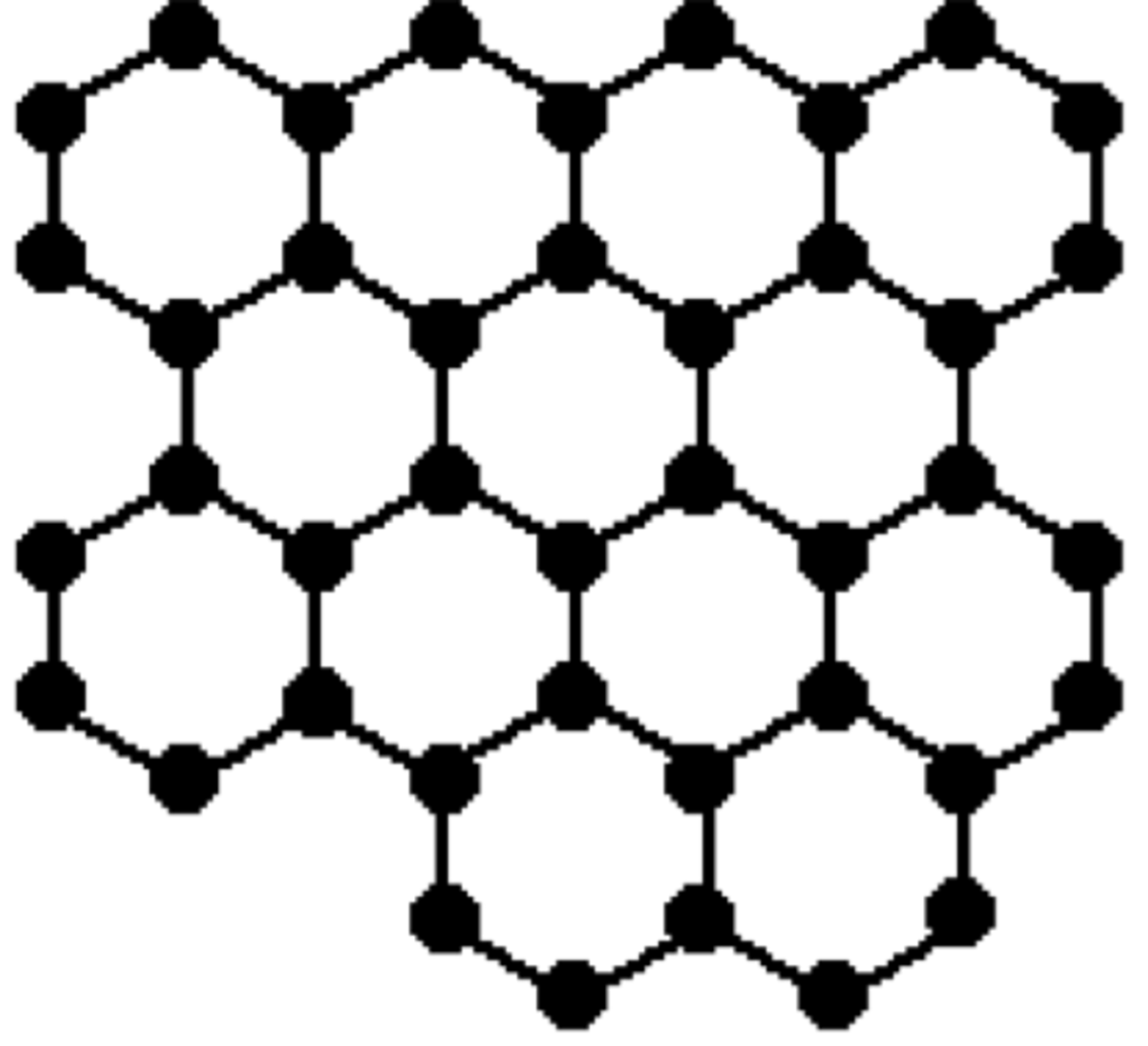
Multiple layers of carbon atoms where each atom is bonded to three other carbon atoms. Layers are have weak attractions between them.
Graphite
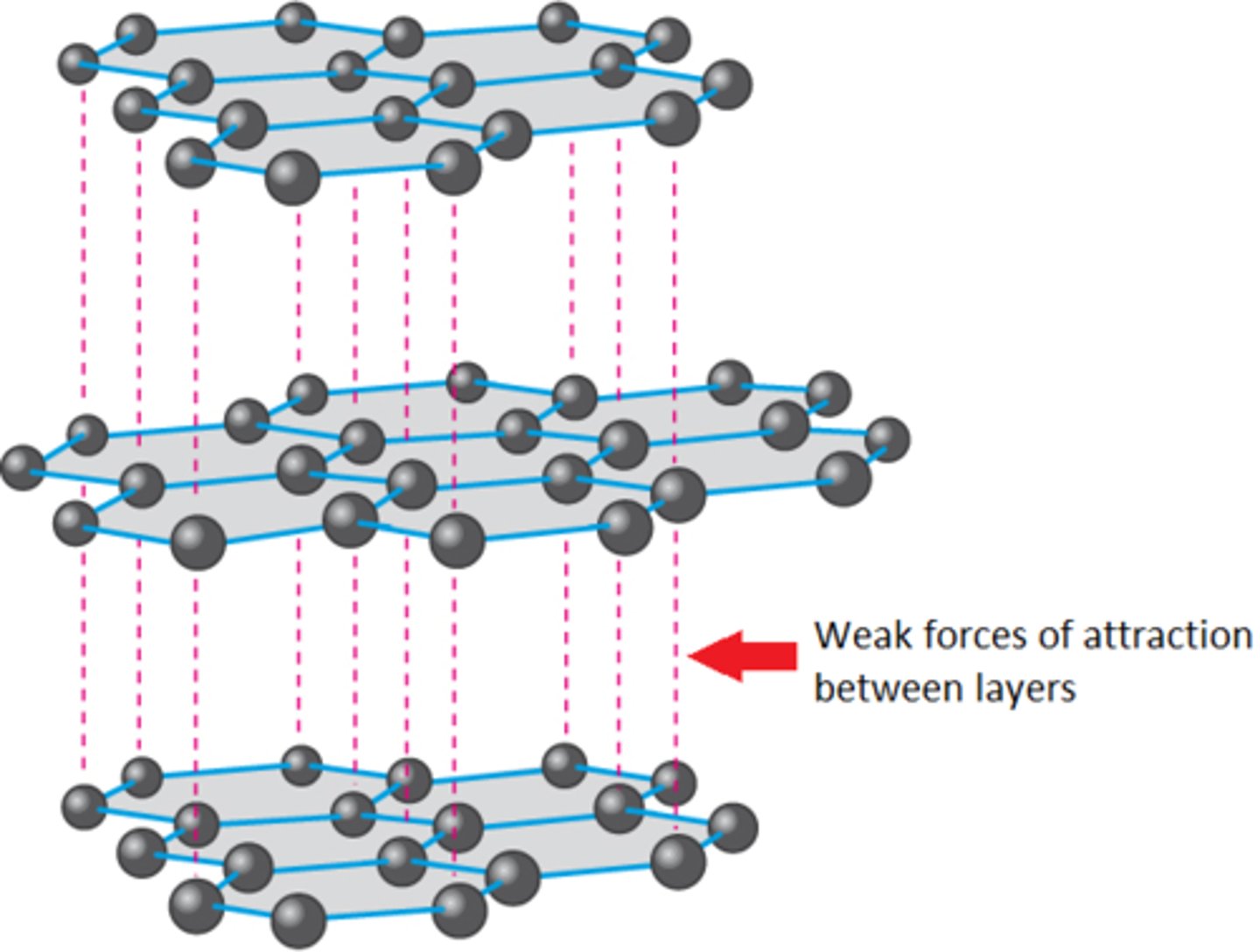
Graphite can be used as a ........................ as the layers slide over each other easily
Lubricant
Graphite conducts electricity because it has...
Delocalised electrons
Diamond does not conduct electricity well because it has...
No delocalised electrons
A common use for graphite
Pencils
Sheets of graphite can slide over one another because they have...
Weak intermolecular forces
Fullerenes can be what shapes?
Tubes or balls
Which giant carbon structure can be said to strengthen tennis rackets?
Carbon nanotubes
Which giant carbon structure may be used to deliver drugs into targeted cells?
Buckyballs (graphene balls)