Introduction to Analytical Separations & Ion Exchange Chromatography (chptr 22)
1/62
Earn XP
Description and Tags
CHEM 310: Foundations of Analytical Chemistry
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
63 Terms
what is analytical separation?
the ability to separate, identify, and measure one or more components from a complex mixture
what is solvent extraction?
the physical transfer of a solute from one phase to another, using the solubility of the solutes and/or compounds involved
miscible
two liquids form a single phase when mixed in any ratio
immiscible
two liquids remain in separate phases
(non polar solvents are usually immiscible with water which is polar)
“like dissolves like”
solute is more soluble in solvent with similar polarity
what is the most common case of an extraction?
the extraction of an aqueous solution with an organic solvent
why would we utilize pH effects on extractions?
when we have a solute that’s an acid or base…
its charge will be based on the pH value of the solvent
neutral species is generally more soluble in organic solvent
charged species is generally more soluble in aqueous solvent
ion-pair extraction
hydrophobic cations and anions can function as ion-pairing agents to bring ions of opposite charge into organic solvents
surfactants
molecules with both hydrophobic and hydrophilic character that accumulate at interfaces between two phases and modify the surface properties
what is chromatography?
has the same principle as extraction, except one phase is held in place while the other moves past it
stronger attractions between column and compound = longer compound stays on column
separates components based on their attraction to column vs. solvent
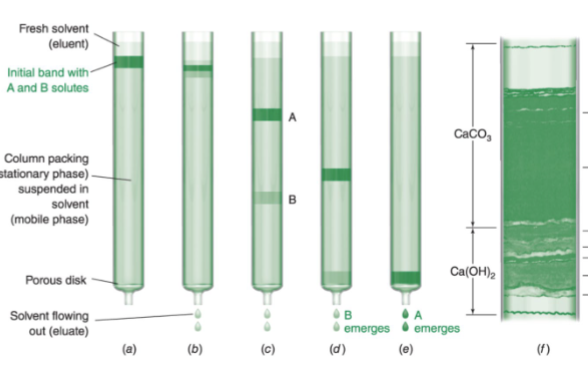
mobile phase
solvent moving through the column as either a liquid or gas
stationary phase
the phase in the column that’s typically a viscous liquid chemically bonded to the inside of a capillary tube or onto the surface of solid particles packed in a column—but it can also be the solid particles themselves inside the column
eluent
fluid entering the column

eluate
fluid emerging from the end of the column

elution
process of passing a liquid or gas through a chromatography column which can be packed or open tubular
5 types of chromatography
adsorption
partition
ion-exchange
size exclusion / gel filtration
affinity
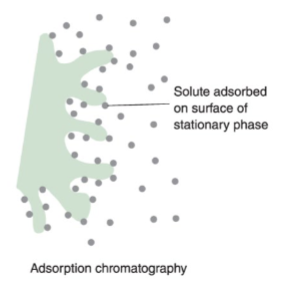
adsorption chromatography
solid stationary phase
liquid or gas mobile phase
solute is adsorbed onto the surface of the solid particles
when a stronger solute is adsorbed, the slower it eluates from the column
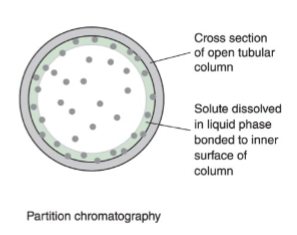
partition chromatography
liquid stationary phase bonded to a solid surface
liquid or gas mobile phase
solute equilibrates between the stationary liquid and the mobile phase
example: gas chromatography
ion-exchange chromatography
solid stationary phase, called resin, has anions or cations covalently attached to it
liquid mobile phase
solute ions are retained by oppositely charged sites on the stationary phase
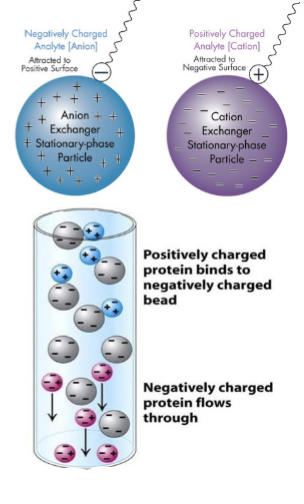
ion-exchange chromatography: anion exchanger
has positively charged resin which attracts anions
ion-exchange chromatography: cation exchanger
has negatively charged resin which attracts cations
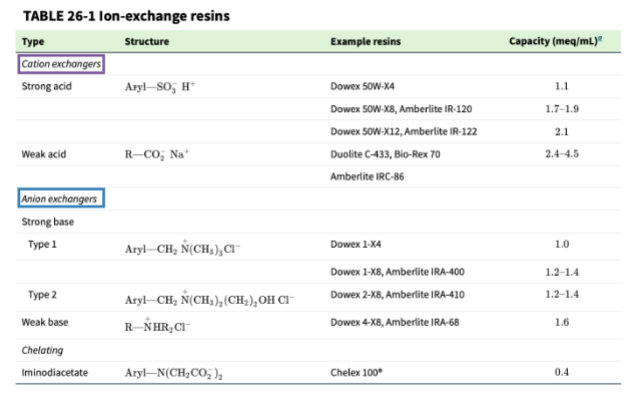
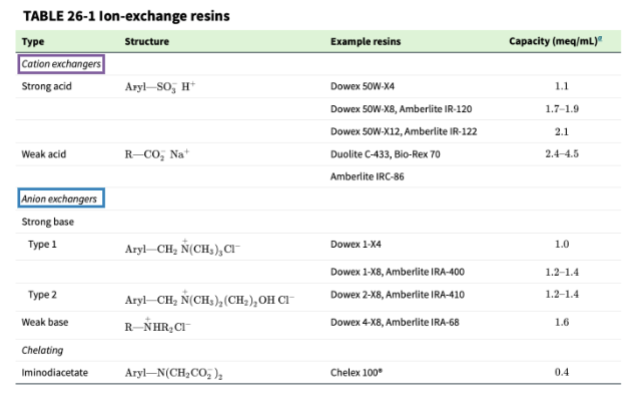
ion exchangers
resins are amorphous (non crystalline) particles of organic material, and chelating resins have a high preference for binding transition metal ions
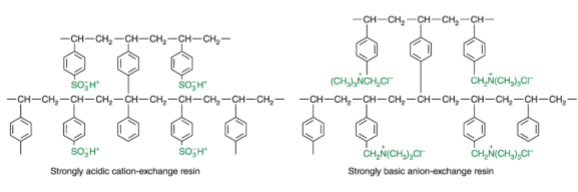
ion exchangers: polystyrene resins
have pore diameters of ~1 nm, whereas microporous resins have diameters of ~100 nm; typically used for proteins and macromolecules
ion exchangers: cellulose, dextran, and agarose
polymers of sugar molecules which possess larger pore sizes and lower charge densities that those of polystyrene resins; because they’re softer than polystyrene resins, they’re called gels

ion-exchange selectivity and equivalents
to consider the competition for Na+ and H+ for sites on the cation exchange resin R+, look at the selectivity coefficient (K), which describes the relative selectivity of the resin for Na+ and H+
selectivity for polystyrene resins tends to increase because…
of the increased extent of cross-linking
generally speaking, ion exchangers tend to favor the binding of ions with…
higher charge
decreased atomic radius
increased polarizability
polarizability
the ability of an ion’s electron cloud to be deformed by nearby charges, resulting in the induced dipole increasing the ion’s affinity for the resin
bind to an ion-exchanger
is an equilibrium process, and the amount of charge exchanged is measured in equivalents (e.g. one mole of Ni2+ exchanges with two moles of H+ means one mole of Ni2+ is 2 equivalents)
ion-exchange capacity
the number of ionic sites on a resin that can participate in the exchange process; listed as either meq/gram of dry resin or meq/mL of wet resin
ion-exchange chromatography: resins vs gels
resins are used for small molecules with a molecular weight of less than 500 g/mol, and gels are used for large molecules like proteins
gradient elution
eluting with increasing strength or changing pH; constantly changing the composition of the mobile phase to increase the concentration of the well-retained solutes
ion chromatography
the high performance version of ion-exchange chromatography; typically used to monitor amounts of specific anions and cations in solution (e.g. how the U.S. EPA determines the ions in drinking water)
size exclusion chromatography
separates compounds based on size
larger solutes elute more quickly
no attractive interactions between the stationary phase and the solute
stationary phase = porous resin
mobile phase = gas or liquid
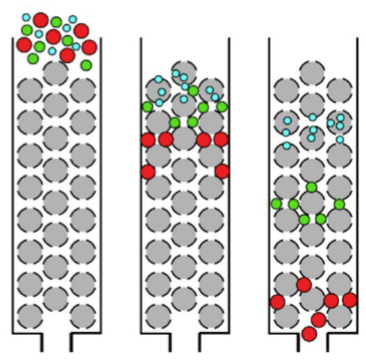
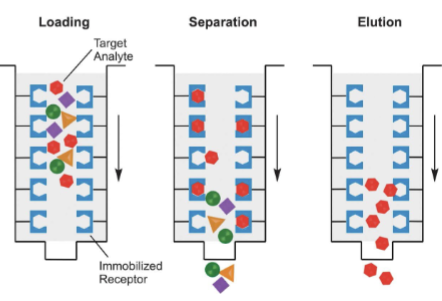
affinity chromatography
most selective because it uses molar recognition between an immobilized molecule covalently attached to the stationary phase, and solute molecules to which it binds specifically
example: used to purify a protein by its unique binding to a specific ligand or antibody bound to resin in column
stationary phase = resin with some immobilized molecules
mobile phase = liquid
volume flow rate (F)
speed of the mobile phase passing through a chromatography column told by how many mL of solvent per minute flow through the column
linear velocity (ux)
speed of the mobile phase passing through a chromatography column told by how many cm are traveled in 1 minute by the solvent; = L/tM’ where L is column length
each cm of column length has a volume of…
𝜋r2 × length
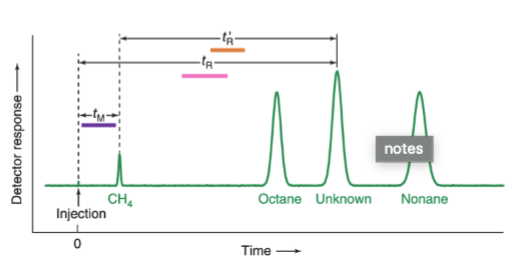
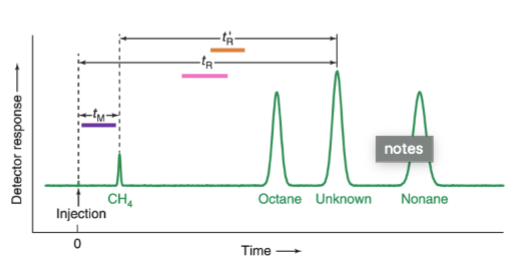
chromatogram
graph showing the detector response as a function of elution time
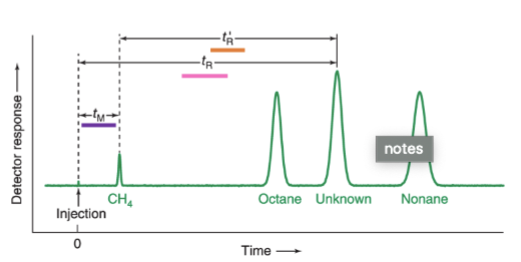
retention time, tR
the time that elapses between injection of the mixture onto the column and the arrival of that component at the detector
retention volume, VR
the volume of the mobile phase required to elute a particular solute from the column
when is the retention time and volume of a compound constant?
under constant chromatographic conditions
is retention time used for qualitative or quantitative analysis?
qualitative
is peak area/peak height, when compared to standards, used for qualitative or quantitative analysis?
quantitative
tM
the mobile phase or unretained solute that travels through the column in the minimum possible time
adjusted retention time, t’R
the additional time required to travel through the length of the column, beyond that required by the solvent; = tR - tM
retention factor, k
the time required to elute a specific peak minus the time tM required for the mobile phase to pass through the column, expressed in multiples of tM
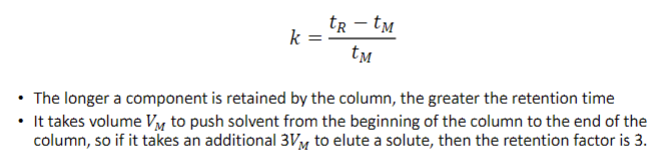
the longer a component is retained by the column, the [lesser/greater] the retention time
greater
It takes volume 𝑉𝑉𝑀𝑀 to push solvent from the beginning of the column to the end of the
column, so if it takes an additional 3𝑉𝑉𝑀𝑀 to elute a solute, then the retention factor is…?
3
separation factor, 𝛼
the ratio of the adjusted retention times for two components used to identify peaks when the flow rate changes

an increase in separation factor means there’s a [low/high] flow between the two components
high
relationship between retention time and the distribution constant
-the retention time volume, VR, is the volume of the mobile phase required to elute a particular solute from the column,
-F is the volume flow rate (volume per unit of time) of the mobile phase
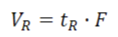
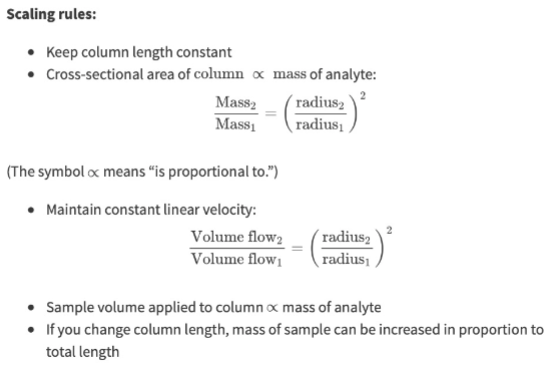
To scale up… (consider column length, analyte mass, radius, linear velocity, sample volume, …)
what are the 2 major factors that the efficiency of peak separation depends on?
the difference in elution times between peaks (you’ve got a better separation when the peaks are further apart)
the broadness of the peaks (you’ve got poorer separation when the peaks are wider)
common measures of peak broadness
width (w1/2) measured at a height equal to half of the peak height (FWHM)
the width (w) at the baseline between tangents drawn to the steepest parts of the peak
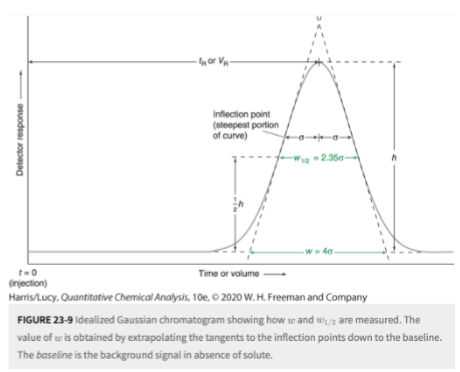
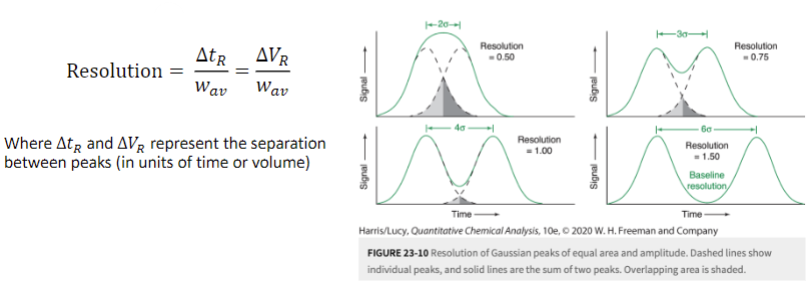
efficiency of peak separation: resolution
how close two peaks can be to one another while still being identified as two peaks
diffusion
a band of solute broadens as it moves through a column
what’s one main cause of band spreading?
diffusion, by the net transport of a solute from a region of high concentration to a region of low concentration caused by the random movement of molecules
diffusion coefficient
is related to the thermal energy of a molecule and the friction it experiences while diffusing
depends inversely on the viscosity of the medium and the size of the diffusing molecule
104 times slower in liquids than gases due to the greater viscosity of liquids
macromolecules are 10-100 times slower than small molecules

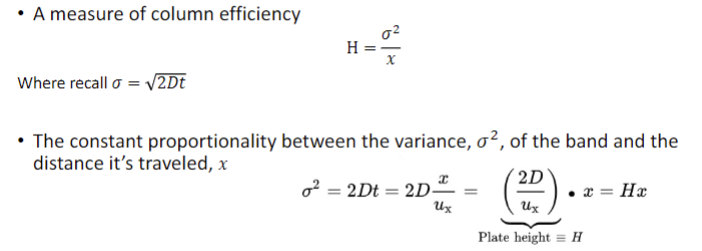
the role of plate height (H) in separations
smaller plate heights = narrower peaks
the role of plate number (N) in separations
solute emerging from a column of length, L, the plate number, N, in the entire
column is the length L divided by the plate height
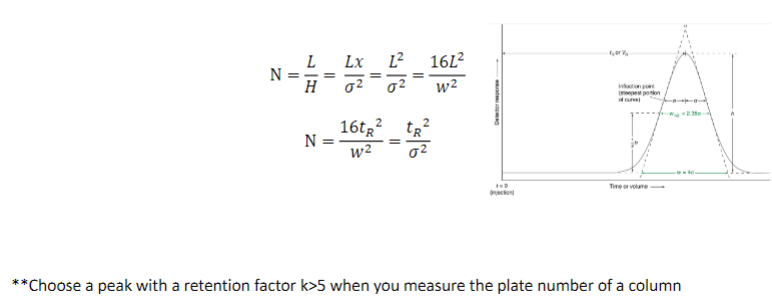
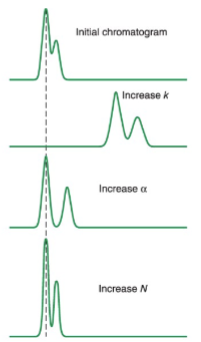
factors affecting resolution of chromatograms: plate height and plate number
resolution is proportional to √N and proportional to √L
doubling L increases resolution by √2
increasing L also increases separation time though
decreasing L will increase N with no change to the separation time
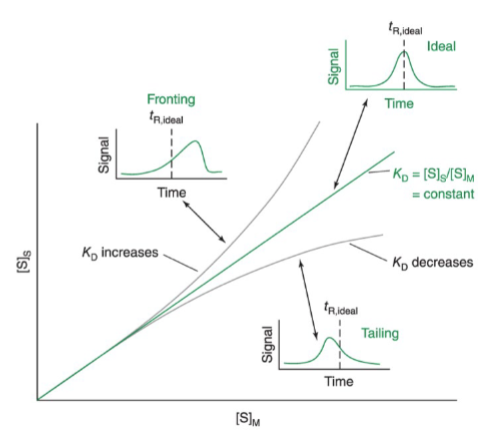
asymmetric peaks on the chromatogram
increased KD results in peak fronting
there is so much solute in the stationary phase that the stationary phase begins to resemble solute
decreased KD results in tailing peaks
the column has a limited retention capacity, and the injection of concentrated solute saturates a significant portion of sorption sites, leaving fewer sites available for retention and making the k lower