Tolerance, Autoimmunity and Hypersensitivity
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
What is tolerance?
Tolerance is an acquired state of unresponsiveness to substances or tissues that are capable of eliciting an immune response.
What is an adjuvant and how do they work?
Strong adaptive response almost always require the antigen to be injected into a mixture known as an adjuvant.
They cue the immune system that an infection is taking place.
They convert soluble protein into particulate material, which is ingested by antigen presenting cells such as macrophages. Some adjuvants contain bacterial products, which stimulate macrophages or dendritic cells through Pattern Recognition Receptors.
How does central tolerance of T cells work?
Clonal selection.
First generate T cell receptors regardless of specificity
Positive selection of the small number of receptors that work with self MHC to foreign antigens.
Negative selection of the ones that bind too strongly to the self MHC plus peptide.
Thymus, negative selection leads to deletion of TCR that are high affinity to self.
What factors affect tolerance?
Timing, dose of antigen, amount of costimulation, location
What is Medawar’s tolerance experiment and what did it show?
Mice A were injected at birth with bone marrow from B.
6 weeks later they were grafted skin from mouse B.
The skin graft from B was accepted whereas graft from mouse C was rejected.
A had acquired tolerance to B.
If B bone marrow were injected a week after birth tolerance was not achieved and grafts of skin B were rejected.
Bone marrow stem cells from mouse B establish chimerism in the host. Some cells differentiate into APCs and migrate to thymus where they tolerise developing thymocytes. Lifelong chimerism needed to maintain tolerance. If transfer is done later, the host is sufficient to destroy the donor stem cells before they can engraft.

What is the evidence for negative selection?
Transgenic mice specific for male antigen expressed from Y chromosome. Class I MHC restricted.
Preferentially promote the development of CD8+.
Female mice - CD8 cells developed.
Male mice - failed to develop = tolerised.
What is AIRE and what is its purpose? What is the effect of a deficiency on an individual?
Some antigens are not expressed until after the immune system has matured, others only expressed in specialised tissues.
This limitation is circumvented by expression of a promiscuous TF called AIRE, which turns on many 'peripheral' genes in the thymus, so that the developing T cells may be exposed to their products.
Individuals lacking AIRE suffer from autoimmune conditions. The condition is called APECED, autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, sometimes also referred to as Autoimmune Polyglandular Syndrome Type 1. APECED/AIRE can live fully, but suffer from autoimmune syndromes as well as candidiasis.

How are B cells tolerised?
B cells that react to abundant antigens on self cells are eliminated as they develop by apoptosis
Self reactive cells have the opportunity to avoid apoptosis by receptor editing - changing light chain of the Ab.
High does of soluble proteins result in anergy rather than apoptosis.
What are the mechanisms of peripheral T cell tolerance?
Ignorance: when potentially self-reactive T cells are not activated because antigens are hidden in locations that are not exposed to surveillance. These sites include the brain, eye and testis and have limited or no lymphatic drainage. Split tolerance can be broken. An example → damage to one eye can expose eye-specific antigens lead to destruction of second eye. Ignorance is not complete and can be breached.
Split tolerance: many immune pathways are interdependent, not all need to be tolerised. EG where T cell tolerance has been established but autoreactive B cells are present. Without T cells B cells are 'helpless'. The explanation is that it takes 100 1000- times more antigen to tolerise B cells than T cells.
Anergy: non-responsiveness. Induced in T cells if the second signal is absent. The anergised cell does not die no longer responds. Immature B cells may be anergised by exposure to soluble antigen.
T-reg suppression: some autoreactive T cells appear to be prevented from reacting by other T cells. Tregs have an intermediate affinity for self and not deleted by negative selection. Tregs suppress proliferation of naive T cells responding to autoantigens presented on APC. Secretion of anti inflammatory cytokines IL-10 and TGF-β contribute to down-modulation. Activation of enzyme (IDO) by DCs inhibits T cell growth by depleting tryptophan, inhibition of proinflammatory cytokines, and signalling to decrease B7 taken up by CTLA-4 - highly expressed by Treg.
T cell exhaustion: T cells fail to clear a chronic infection/tumour. Probably evolved to avoid prolonged and harmful futile T cell responses. Exhausted T cells express PD1 which binds PD-L1 and 2. PD1 suppresses T cell signalling renders less responsive to stimulation with antigen. T cell exhaustion reduces autoimmunity severity.
How does tolerance operate in pregnancy?
The foetus and placenta share child’s genotype with ~50% paternal contribution.
Several tolerance mechanisms:
Physical barrier to the mother’s T cells.
Lack of MHC class I expression. Trophoblast cells that form the outer layer of the placenta in contact with maternal tissues do not express class I so not targets for cytotoxic T cells.
Production of immunosuppressive factors such as α-fetoprotein and IDO (a tryptophan catabolising enzyme).
What happens to people that lack Treg?
Boys with a congenital lack of Treg due to mutations FOXP3 die by 2 unless treated due to lymphoproliferation and autoimmunity.
This X-linked syndrome is called IPEX. Scurfy mice lack Foxp3 and only survive for 4-6 weeks. In both cases, effector T cells undergo massive proliferation and cause lethal autoimmunity. Treg are therefore an essential mechanism of self-tolerance.
What do exhausted T cells express?

How can we use the theory of T cell exhaustion/ tolerance to provide cancer therapies/arthritis?
Abetacept is form of CTLA4. Because CTLA4 binds B7.1 and B7.2 with greater affinity than CD28, injection of Abetacept prevents T cells from receiving their costimulatory signal. Abetacept treats rheumatoid arthritis and prevent graft rejection.
Antibody against CTLA4 (Ipilimumab) which prevents binding to CD80 or CD86. This breaks tolerance and enhances CD28. Ipilimumab is approved for metastatic melanoma. It works by activating a strong immune response against neoantigens expressed by the cancerous melanocytes.
Anti-PD1 or anti PD-L1. PD1 is expressed by activated T cells, and like CTLA-4, delivers a negative signal to the T cells. PD-L1 can be expressed by the tumour cells and by macrophages in the tumour microenvironment.
What is the hygeine hypothesis and what is the cryptic infection hypothesis?
Hygeine hypothesis: increase in autoimmune conditions in developed countries is that the immune system is not conditioned by exposure to infection - supported by the realisation that autoimmunity has increased as infections such as measles, mumps and TB have declined in incidence.
Cryptic infection hypothesis: exposure to diverse commensals (‘friendly’ bacteria) that comprise your microbiome prime the immune system. It is possible that some autoimmune conditions are caused by infectious organisms not identified.
What are the common organs affected by autoimmunity and what are the typical immune responses?
In autoimmunity the immune system seems to be attacking cells as if they were infected.
antibodies (autoantibodies)
T cells (autoimmune T cells) to autoantigens on target tissues.
Common organs include thyroid, adrenals, stomach and pancreas - well vascularised and make organ specific proteins. Non-organ specific targets include the skin, kidney and joints
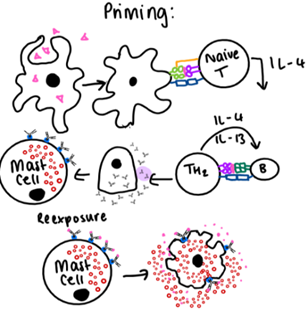
Describe Type 1 Hypersensitivity and give examples.
1st encounter: antigen taken up by DC
Recognised by T cell
T cell reacts, clonal expansion, activates B cells that differentiate to plasma cells.
Plasma cells release IgE.
IgE binds to mast cell which makes it primed.
2nd exposure: antigen binds to IgE on mast cell.
Mast cell degranulates releasing histamine.
Vasodilation and increased vascular permeability
Atopy, asthma, allergy, anaphylaxis
Describe Type 2 Hypersensitivity and give examples.

IgG or IgM recognise the antigen and bind on the cell.
The opsonisation activates the complement response.
Can also activate the NK cells and cytotoxic cells that do ADCC releasing substances like perforin which induce apoptosis in the target cells.
Death of cells
Debris swept up by macrophages and also red blood cells.
Rh ABO incompatability, good pasture syndrome, Ab against collagen (kidney and lung - autoimmunity), haemolytic anaemia, kill RBC
Describe Type 3 Hypersensitivity and give examples.
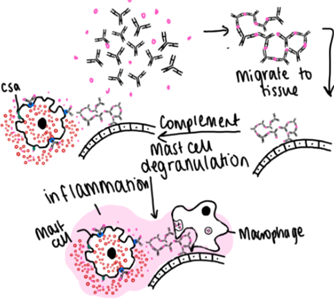
IgG binds to antigen forming a complex.
These aggregate
Complex deposits in tissues on the basement membrane.
Complement is activated via C5a in the tissue - priming the mast cell.
Mast cells can release cytokines by degranulation leading to inflammation.
Local inflammation and phagocytosis
Post strep glomerulonephritis, SLE (2 and 3), antigen is DNA or RNA → damage lots of cells. Type 2 part as some Ag against RBCs, Kidney or joints is target. Serum sickness - Ab from horse, Hepatitis B and C and Malaria
Describe Type 4 Hypersensitivity and give examples.

Antigen taken up by DC.
DC processes and presents as MHC-1.
T helper 1 cell binds and becomes primed.
T helper cell clonal expansion
Second exposure: T helper cell fast response, releasing cytokines and maybe activated CD8 cells.
Macrophage activated by cytokines and directly removes the target cells.
Contact dermatitis, Type I Diabetes, MS (myelin presents to B cells, epitope spreading), R. Arthritis (self - anti TNF a Ab), TB (infectious)
What is relative risk and how can genetic and environmental factors predispose people to autoimmune disease?
Most autoimmune diseases are associated with one or more HLA allotype. These are not encoded by ‘mutant’ alleles but are polymorphic variants in the normal population. A common way of expressing the way allotypes influence disease is the concept of Relative Risk.
What factors can predispose people to auto immunity (x3)?
HLA allotype - certain haplotypes associated with a number of autoimmune diseases like Graves and SLE. Multiple alleles contribute to suceptibility, most autoimmune conditions have an MHC association.
Other genetic factors - Genome wide association studies show that multiple genetic loci are involved in susceptibility such as diabetes and rheumatoid arthritis, multigenic disorders
Sex differences - not equal in both sexes, increased susceptibility may be due in part to hormonal differences and in part due to genes found on the X chromosome which are imperfectly silenced - females express higher. One gene TLR7 may promote susceptibility to Lupus.
Environment - twin concordance rate is about 20-40%. Childhood infection or exposure to microbes, but are protective or predisposing. Rheumatic fever can follow Streptococcal infection; reactive arthritis after Shigella or Chlamydia. Non-specific infection is known to cause a flare-up of MS. The MHC contributes about 25% and the other 25% comprises a variety of other genes.
How can autoimmunity be triggered by infection?
Release of sequestered antigen e.g. autoimmune sympathetic ophthalmia, damage to one eye leads to subsequent attack of other eye
Molecular mimicry - if an antibody can also cause an autoimmune response - cross reactive. The potent immune response elicited by the infectious agent may hence break immunological tolerance. A similar scenario can occur with T cells. Rheumatic fever is caused by cross-reactive antibodies to Streptococcus.
What is the trigger and mechanism of Rheumatoid arthritis?
Rheumatoid arthritis (RA) may involve protein modification – in this case protein citrullination by peptidylarginine deiminase
Initially this may occur in the lung and there is a strong link between RA and smoking. Antibodies to the modified proteins: ACPA (anti-citrullinated protein antibodies) are present in most patients with RA.

What is the trigger and mechanism of Coeliac disease?
Coeliac disease is not strictly autoimmune as it is dependent on eating wheat. It involves presentation by specific HLA-DQ molecules of deamidated gliadin peptides. Removal of the antigen from the diet is generally curative.

What is the trigger and mechanism of Graves Disease?
Graves disease: This is due to antibody to TSH receptor. Unlike the natural ligand, TSH, the antibody is not subject to feedback inhibition, resulting in overproduction of thyroid hormones and hyperthyroidism. Graves may be a ‘Th2 type’ response, in which there is little inflammation or lymphocyte infiltration.

What is the trigger and mechanism of Hashimotos thyroiditis?
In contrast, Hashimoto’s thyroiditis is mostly a Th1 response as CD4 and CD8 T cells infiltrate the organ. Antibodies are also generated against thyroid peroxidases, thyroidglobulin and THS receptors (in contrast to Grave’s disease, antibodies against the TSH receptor are blocking in Hashimoto’s disease). Hashimoto’s thyroiditis is much more common in women than men.
What is the trigger and mechanism of Myasthenia Gravis?
In Myasthenia Gravis autoantibodies to the acetylcholine receptor diminish neuromuscular transmission from cholinergic neurons by blocking the binding of acetylcholine and by causing downregulation (degradation) of its receptor.

What is the trigger and mechanism of SLE?
In SLE, patients have a wide variety of anti cytoplasmic and anti-nuclear auto-antibodies. The condition results in a characteristic 'butterfly' or ‘wolf’ rash on the face. There can be significant depletion of complement in these patients. In addition, complement deficiencies that impair immune clearance, such as C1, C2, C4 are predisposing factors. SLE is much more common in women than in men.
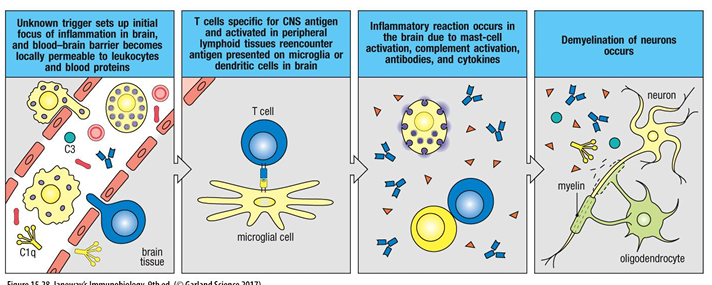
What is the trigger and mechanism of MS?
MS is caused by T cells that cross the blood-brain barrier and damage the myelin sheath that surrounds the nerves. This leads to impaired nerve transduction and hence impaired mobility and other neurological effects.
What is the trigger and mechanism of Type 1 Diabetes?
Type 1 diabetes (T1D) is caused by T cells that attack the insulin-producing β cells in the pancreas. Insulin stimulates glucose uptake by muscles, liver and fat. As a consequence of β cell destruction, blood glucose levels can become dangerously high, causing neurological and vascular damage. Those affected by type 1 diabetes 14 therefore need to monitor glucose levels in inject insulin as needed. There is no cure, but research is focussed on transplantation of βislets as well as various strategies to induce immunological tolerance towards the β cells
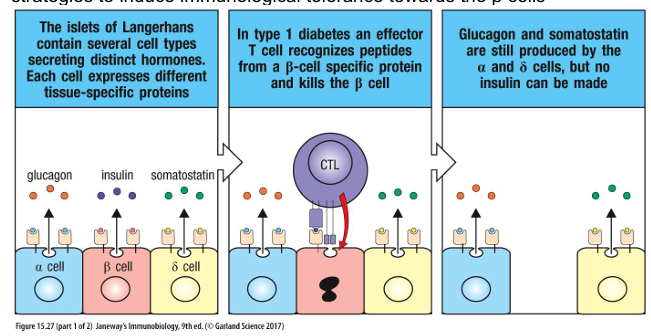
What are the three mechanisms of transplant rejection? Why does transplantation lead to rejection?
Hyperacute
Acute
Chronic
The main problem with transplanting tissue is that most cells express polymorphic surface antigens encoded by the MHC. Variation between the donor and recipient at the MHC results in rejection. A central role for recognition of transplanted tissue is played by T cells but other cells may be involved in rejection, including NK. However, in some cases antibodies are important, although their production may be initiated by T cells. T cell recognition may be through direct recognition of the donor MHC or indirect recognition, of an antigen presented by self MHC molecules.
What does allogenic, iatrogenic mean?
Allogenic cells - cells from a different host
Iatrogenic - effects of medical treatment
What are the 4 different kinds of transplantation?
Autologous - from same animal to itself
Syngeneic - from animal of same species closely related to the other, like family member
Allogenic - same species but not related
Xenogeneic - different species
Why is rejection to a second skin graft from the same donor more rapid than the primary rejection?
The immune system is primed by the allograft, upon first encounter.
When a recipient that has previously rejected a skin graft is regrafted with skin from the same donor the graft is rejected more rapidly, in a second-set reaction.
Skin from a third-party donor grafted onto the same recipient would be rejected as for the first-set.
The rapid second set rejection course can be transferred to normal or irradiated recipients by T cells from the original recipient. This shows that second-set rejection is caused by a memory immune response from clonally expanded and primed T cells specific for the donor skin. Memory T cells are produced alongside effector T cells in a primary immune response. Upon a second exposure to the same antigens memory T cells promote a more rapid, more effective response. This is because memory T cells to a specific antigen are more numerous than naïve T cells and they are more readily activated.
When does the hyperacute rejection response occur?
From previous organ transplants (e.g. children who have multiple transplants)
From pregnancy – at childbirth foetal cells enter maternal circulation and stimulate adaptive response to paternal HLA
From blood transfusion (matched for ABO but not HLA)
Xenotransplants - recognising glycosylated proteins. We make natural IgM and IgG to modified sugars on pig. Second, complement does not function well across species. Normally complement is disabled on self by action of regulatory proteins such as decay accelerating factor. This does not work on pig tissue and the graft is attacked by human complement.
How does hyperacute rejection work?
The tissue is rejected rapidly as ABO and HLA antigens are expressed on the endothelial cells lining blood vessels. The tissue is damaged by complement activation, coagulation and leakage of fluids, as well as by aggregation of platelets that block the microvasculature.

When does acute graft rejection happen?
Allotransplantation
Not an issue for blood transfusion as RBCs do not carry MHC antigens.
Type of Hypersensitivity Type 4 reaction as it involves CD8 cells to HLA 1 and CD4 to HLA 2.
How does acute rejection work? (2 modes of recognition)
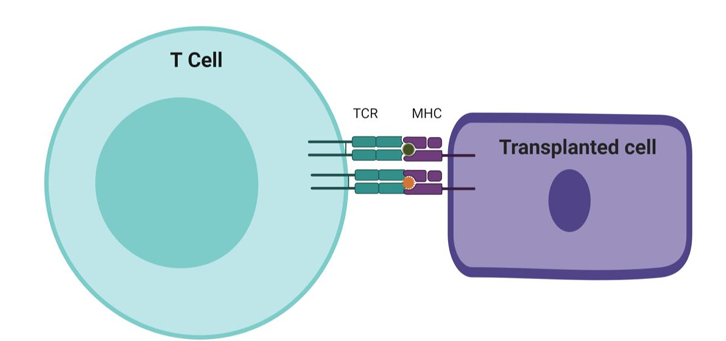
Direct recognition of allo MHC - Most organ transplants are not perfectly MHC matched → alloreactive T cells that react to non self MHC in transplanted tissue. Some are memory cells that once recognised pathogens but can also react with the transplanted tissue. TCR can bind strongly to multiple allogenic MHC. No central tolerance against all MHC. Normally only a few MHC on cells would present as foreign peptides but on allogenic cells every MHC = target. CD8 and CD4 mediate graft rejection.
Indirect recognition - uptake of allogenic proteins in APCs and presentation to T cells by self MHC.
Why do the products of the MHC cause such reproducible and rapid graft rejection - acute rejection? How does direct recognition work?
Naive individuals inadvertently have a high frequency of TCRs reactive with allo-MHC.
Negative selection in the thymus reduces the number of T cells that see self antigens but does not do alloantigens.
These receptors do not need to bind with high affinity to activate the T cell. Unlike normal antigen presentation, T cells in transplants could engage every MHC ligand on the cell. The recipient is sensitised by migration of allogeneic DCs from the inflamed donor organ via lymph to host's secondary lymphoid.
DCs settle in T cells zones and present donor MHC to activate those host T cells bearing the corresponding TCRs. The alloreactive T cells are carried back to the graft, which they attack through the direct pathway of allorecognition.
The effector T cells migrate to the transplanted tissue and Th1 T cells activate macrophages → inflammation → CD8 cytotoxicity.
How does indirect recognition by acute rejection work?
Uptake of allogeneic proteins by recipient’s own APC’s and presentation to T cells by self MHC.
Donor DCs migrate to secondary lymphoid and die by apoptosis or necrosis.
Graft-derived peptides presented by recipient’s APC’s = non-MHC ‘minor’ or H antigens.
Both HLA and non-HLA proteins different from host are taken up by DCs and processed so peptides are presented by recipient’s HLA. endocytosis → HLA class II. → stimulate a CD4 T cell alloreaction.
Thus, in principle T cells activated this way are specific for self-HLA molecules plus donor peptides → destruction of the graft.
Some T cells will cross react with donor HLA plus peptide. Counter-intuitively, MHC sharing between donor and recipient increases reactivity as DCs can prime recipient T cells for minor peptides.
Other mechanisms include activation of macrophages, produce lytic enzymes, production of Ab through B cell activation and immune complex formation and ADCC.

Why is indirect rejection slower than direct in acute?
Rejection is slower than for direct recognition although many MHC minor differences combine to give rapid rejection. In experimental animals even grafts between animals of the same isogenic strain can be rejected, for example where the donor is male and the recipient female and the H-Y antigen is recognised. In human transplantation recognition of male minor antigens can play an important part in haemopoietic stem cell transplants.
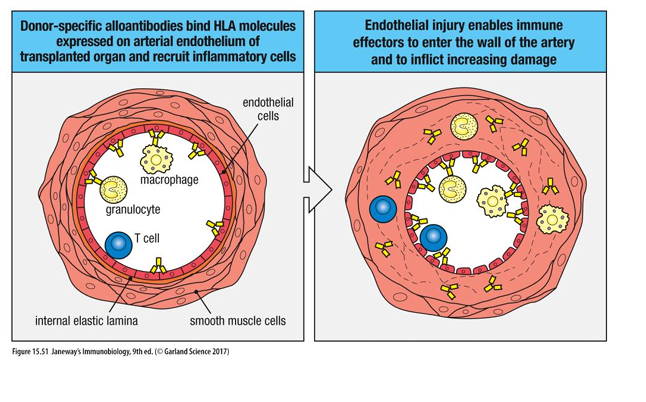
How does chronic rejection work?
Rejection of matched organs can occur years after transplant.
The mechanism is obscure but may relate to immune response against blood vessels. The blood supply to the organ is compromised, resulting in ischemia and loss of function.
Chronic rejection may be due to type III hypersensitivity due to IgG antibodies against allogeneic HLA class I on graft, forming immune complexes that deposit in the blood vessels of transplant.
Also, indirect allo recognition is important for chronic rejection as B cells binding donor HLA proteins are ‘helped’ by T cells.
Kidney damage can also affect loss by non-immune mechanisms.
In privileged sites, vascularised solid organs and haemopoietic stem cells is the risk of rejection higher or lower?
Privileged sites - little or no immune rejection. eg cornea since absence of lymphatic drainage and some other sites lack vascularisation = good for rejection.
Vascularised solid organs - pancreas, heart, lung, liver and kidney - HLA matching significant as highly vascularised.
Haemopoietic stem cell transplants - very important for matching as can form blood cells and immune cells so would have double down effect if rejected.
Most important to match HLA-A, -B, -C, -DR
How is HLA matching determined?
Tissue typing: mAbs against MHC alleles → lysis of targets with complement
DNA typing → Genetic screening
Mixed lymphocyte reaction → mixing leukocytes from recipient and donor, detection of preformed Ab against MHC.
Microcytoxity → sera with known anti-HLA antibodies that specifically recognize allomorphs of particular HLA loci. These sera were taken from multiparous women who had produced antibodies to their babies, now monoclonal antibodies. Donor's blood cells HLA typed by mixing them with a panel of such sera. If the antibodies recognized their epitope on the MHC, in the presence of added complement the cells would be osmotically lysed and would take up a dye.
What is crossmatching?
An exact match may be difficult and DNA typing may miss alleles where the gene is present but the protein is not expressed.
It is important to determine if there are any preformed antibodies to potential donor HLA
A positive cross-match indicates that recipient has antibodies against HLA proteins donor. The most common method is to screen the recipient serum against panels of microbeads, each coupled with a specific HLA protein

Why do we need immunosuppression and what drugs may be given, when, why and how do they work?
Immunosuppression is essential for transplantation of organs such as kidneys (excluding privileged sites or autologous).
Some drugs given before, to condition the immune response. These include: Steroids such as prednisone - given for systemic immunosuppressive effects. Steroids have multiple mechanisms of action which mimic those of corticosteroids. They can have severe side-effects when used in high doses.
Humanised antibody to CD52 on the surface of leukocytes results in long-lasting lymphopenia due to activation of complement.
Other drugs given after: Cytotoxic drugs (azathioprine) lead to death of rapidly dividing cells (T cells)
Immunosuppressive drugs that target cell signalling pathways in lymphocytes e.g. cyclosporine, FK506, rapamycin. They block signal transduction for activation of T cells. They need to be maintained indefinitely.
Haematopoietic transplants: what are they used for?
Used to treat genetic immunodeficiencies, leukaemias and lymphomas.
The host’s immune system is ablated (myeloablative therapy) by chemotherapy and radiation and the patient’s immune system is reconstituted with donor cells by liquid therapy.
When is allogenic and autologous haematopoeitic transplants used and how do they work? What are the issues with both?
In Allogeneic transplants the donor is healthy and HLA-matched. The recipient’s immune system is destroyed by irradiation and drugs. Problems arise as donor T cells respond to the recipient’s HLA molecules in a graft versus host reaction - systemic type IV hypersensitivity reaction. T cells from the donor access recipient lymphoid tissue, interacting with host DCs divide and proliferate. These T cells attack epithelial tissues inflamed by irradiation and chemotherapy.
Autologous transplants help to overcome histo-incompatilibity. In this situation, self cells, which are of course perfectly histocompatible, are removed from the patient and any residual immune cells and leukaemia are ablated. The immune cells are then reinfused into the patient after removal of the tumour cells. A problem with autologous transplants is potential return of the cancer (relapse).
Bone marrow transplants can cause graft versus host disease if the infused bone marrow cells contain NK cells and/or T cells. However, such cells can also help kill remaining leukaemic cells through graft versus leukaemia (GvL).

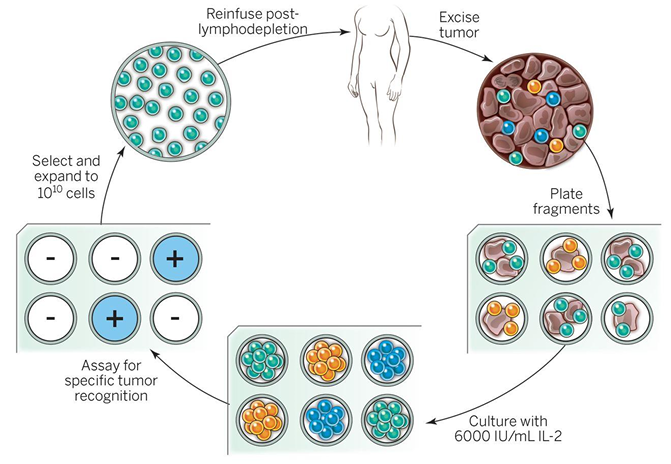
How does adoptive T cell therapy/ CAR-T therapy work?
Adoptive T cell therapy involves the isolation of tumour-reactive T cells which are expanded in cell culture before transferred back into the patient. This remains an experimental form of therapy.
T cells can also be isolated and transduced with a Chimaeric antigen receptor (CAR). CARs have an Ag binding domain derived from an Ab on extracellular side and TCR and costimulatory signalling domains on the intracellularly. CAR-T cells are now approved for the treatment of B cell lymphomas. CAR- T cells for other types of cancers are also under development → novel class of “living drugs”. Effective against B cells as they have unique CD19 and are dispensible - more challenging for other cancers.
What are the therapeutic approaches to prevent graft rejections?
Cyclosporin and Rapamycin are commonly used to prevent graft rejections.
CTLA4-Ig: prevents CD28 costimulation and may induce tolerance toward the graft.
Belatacept is approved for the prevention of kidney transplant rejection.
Tregs - can be expanded and infused back into the patients with the aim to provide tolerance against a graft. This form of therapy has not been effective in the clinic.
Autologous stem cells – regenerative medicine Technically challenging – may in future be a source of artificially grown autologous organs/tissues.