AChem Unit 4 - Electrochemistry
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
What is the nature of electrochemistry?
charged particles; electrons and ions
charged interfaces
oxidation/reduction
electrochemical cells
interfacial reactions
Oxidation
loss of e (D → D+ + e-)
Reduction
gain of e (A + e → A-)
Charge is in units of
C
Faraday’s Law
Q = nFN (n = num electrons, N = nom moles oxidized/reduced, F = Faraday’s constant)
Current is in units of
ampere (A = C/s)
Current is equivalent to _____ in electrochemistry
reaction rate
Potential is in units of
V (J/C)
E_cell develops bc of the
thermodynamic tendency of D to donate electrons
Electrode is a continuum of
energy levels, tunable source of sink/source for electrons
Molecule is
discrete energy levels (some occupied, some vacant)
If E_apl is negative, the electrode is the
reducing agent
If E_appl is positive, the electrode is the
oxidizing agent
Half-cell reaction
reaction of oxidized (e poor) species w e from electrode to give a reduced (e rich) species
Redox couple
oxidized and reduced forms of redox-active species considered together
Trends for O+ + ne- ←→ R
More negative potential means R is good electron donor and reducing agent
More positive means O is a good electron acceptor and oxidizing agent
Why does current flow through a conductor?
charge carriers move in an electric field
Ohm’s law
I = E/R
Resistance depends on
material and geometry
Conductivity and resistivity are intrinsic properties of
electrolyte
Conductance and resistance depend on
electrolyte and geometry
R =
rho*L/A, 1/kappa * L/A
Kappa is the symbol for
conductivity
Resistance in microdisk electrode
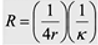
Resistance in microsphere electrode
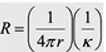
Resistance for electrolyte in long tube
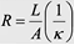
Resistance in parallel planar electrodes
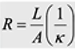
Interphase
electrode surface and adjacent layer of ions (double layer); 1-100 nm thickness
Electrode and electrolyte are both
uncharged
When potential is applied to the electrode, the electrode develops a slight
surface charge and slight local excess of ions develops near the electrode to compensate the charge
Interphase acts as a _______ since it stores charge
capacitor
C =
Q/E
Why is double layer capacitance important
current must flow to charge the double layer whenever the potential at an electrode is changed
Faradaic current is associated with
oxidation/reduction reactions
Non-Faradaic current is associated with
charging the double-layer capacitance
Non-Faradaic current is the _____ in electroanalysis
noise
Discrimination of Faradaic current from non-Faradaic current often sets the ______ in electroanalysis
detection limit
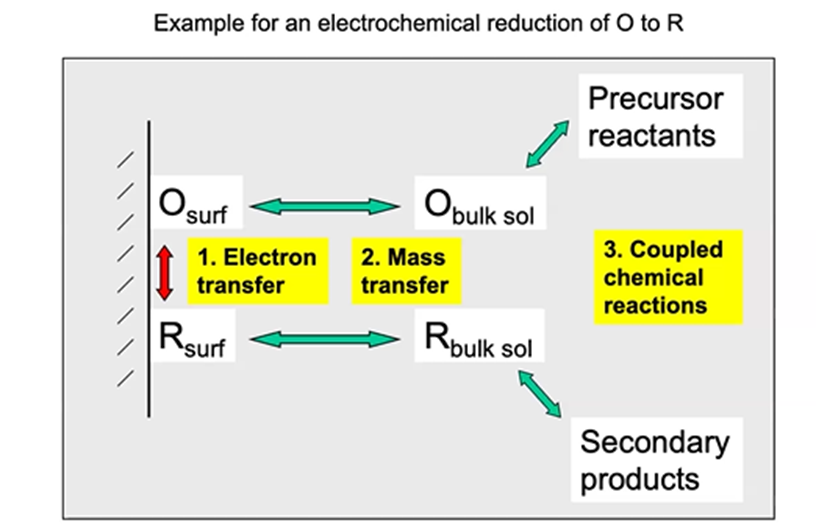
know this
reduction of O to R
Electron transfer
rate depends upon applied potential and molecular and electrode surface structure
Applied potential effects
modest negative potential is slow rate, extreme negative potential is fast rate
Electrode structure effects
Hg electrode, no surface-stabilized intermediates → slow rate
Pt electrode, has surface-stabilized intermediates → fast rate
Mass transfer is usually the factor that limits current when
ET is fast
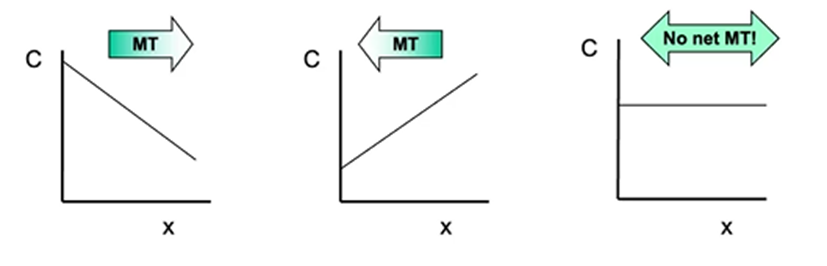
Modes of mass transfer
diffusion
migration
convection
Diffusion
high to low conc
Migration
high to low potential
Convection
high to low pressure
Factors affecting MT
redox species structure and charge
medium viscosity
cell/electrode geometry
hydrodynamics (stirring)
Chemical reactions
bond breaking and making, involved in all but the simplest of electrode reactions
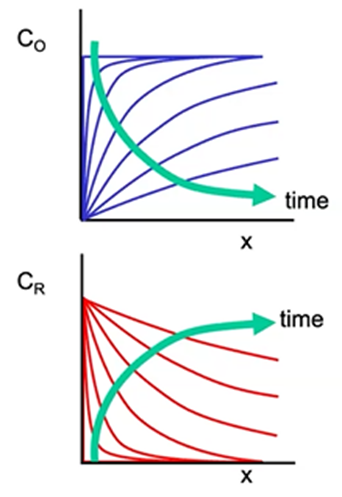
Mass transfer by diffusion
How do C0 and Cr near the electrode respond to the potential step at t = 0?
O is consumed near electrode surface → depletion layer of O grows over time
R is generated near electrode surface → accumulation layer of R grows over time
Flux
rate of MT; dN/Adt = -D(dC/dx)|x=0
D
diffusion coefficient, 10^-5 to 10^-7 cm²/s for small molecules in typical solvents
deltaC0
difference bt C0 at electrode surface and that in the bulk solution
xD
diffusion layer thickness, distance over which C0 and Cr are perturbed due to electrode reaction, 1-100 micrometers
I
-[nFAD/xD]*deltaC0
What about very small electrodes smaller than xD?
large electrode linear diffusion, small electrode radial diffusion
How much stirring is needed to affect MT to electrodes?
enough to shrink diffusion layer → stir solution, flow solution past electrode, rotate electrode
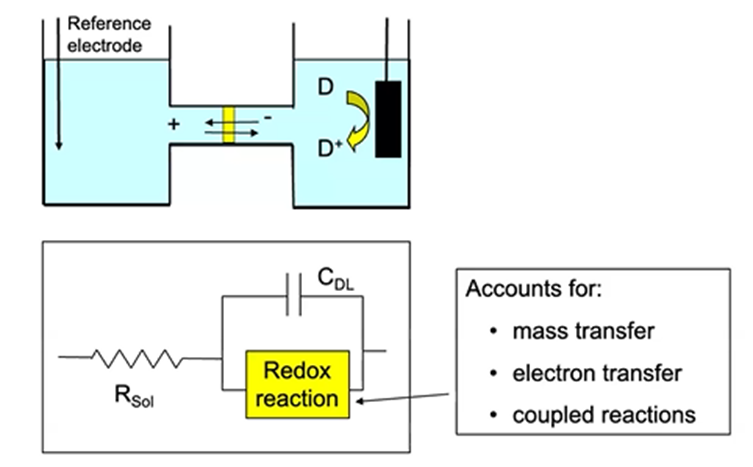
Equivalent circuits
representation of cell by group of electrical circuit elements
Double layer capacitance
electrode/solution interphase
Electrode
solution resistance
Rate of electron transfer
charge-transfer resistance
Amount of reaction
redox pseudocapacitance
In the case of no redox reactions
interphase is capacitor, resistor is electrolytes
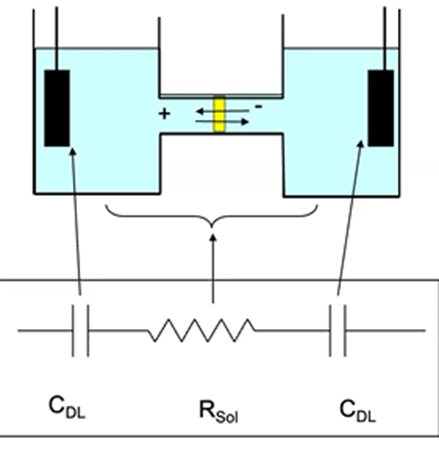
What limits the timescale on which electrochemical measurements may be performed?
cell time constant
Case of redox reaction occurring at working electrode
Practical aspects of electrochemical experiments
reference and working electrodes
solvents and electrolytes
instrumentation
Electrochemical measurements and techniques
potentiometry (ion-selective electrodes)
potential step techniques (chronoamperometry)
potential sweep techniques (voltammetry)
combined sweep and step techniques
sinusoidal techniques (impedance)
Why are reference electrodes needed?
to establish a reliable basis for measuring/applying potentials
E_membrane depends on
membrane and ions in contact w membrane
Working electrodes
electrode where region of interest occurs
Desirable attributes of working electrodes
stable in air/water/solvents
wide potential window (electrode not easily oxidized/reduced, not catalytic for oxidation/reduction of solvent)
well-defined and reproducible surface chemistry
Most common materials for working electrodes
noble metals
other metals
carbon in many forms
Common working electrode configurations
embedded disk
embedded microwire
mercury drop
thin-layer flow cell
thin-film cells
high efficiency flow through cells
rotating disk/ring-disk electrodes
interdigitated electrodes
Non-aqueous solvents…why?
solubility/temperature/acid-base chemistry/water reactivity/chromatography/others
Issues in choosing solvent
liquid range
vapor pressure
polarity/dielectric constant
viscosity
reactivity
toxicity, cost, etc
Issues in choosing electrolyte
solubility in solvent
dissociation in solvent
conductivity
reactivity
toxicity, cost, etc
As you increase pH (become more basic), potential becomes more
negative
Does the pH trend also apply to organic solvents?
not necessarily
Amperometry procedure
apply potential to WE relative to RE and measure current at WE
What is the problem with two-electrode amperometry?
current flows through RE, which can change the composition and potential to change → original assumption is wrong
Potentiostat
allows current through AE and WE but not WE and RE, maintains applied voltage between RE and WE
Important note on potentiostats
reference electrode must always be connected
AE should be large
all 3 electrodes should be physically close together
Amperometry techniques
potential sweeps
potential steps
potential steps and sweeps combined
sinusoidal potential variation
signal vs time techniques
The reduction rate of cyclic voltammetry is faster as you get
more negative
For a potential sweep, peak current is proportional to
concentration, (scan rate)^.5
What two things can happen during a linear sweep voltammetry scan?
C0 at the electrode changes from C0* to 0
Depletion layer of C0* gradually becomes thicker
In cyclic voltammetry, can you measure just the Faradaic current?
no you have to measure Faradaic and non-Faradaic together
What does cyclic voltammetry tell you?
redox potentials and kinetics, coupled chemical reactions
CV is simple to
implement and understand
Why not use CV exclusively?
suffers from poor analytical detection limits (mostly from non-Faradaic current)
For a potential step in chronoamperometry, current is proportional to
concentration, (time)^-.5
In chronoamperometry, there is an increase in diffusion layer thickness over
charge
In chronoamperometry, the current decays exponentially with time, and this is usually much faster than that due to
diffusion limited mass transfer for Faradaic currents
Why use pulses instead of sweeps?
by selecting the pulse periods and delays properly, one can de-emphasize the non-Faradaic currents and emphasize Faradaic currents → improves detection limits
Differential pulse voltammetry
charging currents further diminished
response peak not sigmoid
C_analyte proportional to deltaCurrent_peak
Concentration detection limits near 10^-10 M
Stripping analysis
two step process to analyze for trace metals
preconcentrate metal ions by reduction into Hg electrode
detection by voltametric oxidation of metals from Hg electrode
detection limits as low as 10^-12 M
Why are AC methods used?
studies of electrolyte properties
fundamental studies of kinetics/dynamics of electrode reactions
Frequency = experimental timescale
discrimination against non-Faradaic currents in electroanalysis
Frequency-selective detection filters noises
Phase-selective detection can minimize non-Faradaic currents and enhance Faradaic currents
Higher-harmonic detection can minimize contribution from background currents