CH204 - Test 1 Rxns
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
HX addition to Alkenes
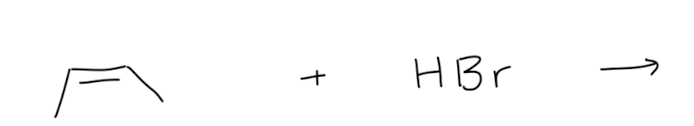
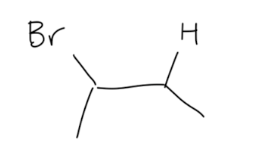
1) resonance stabilized
2) more substituted
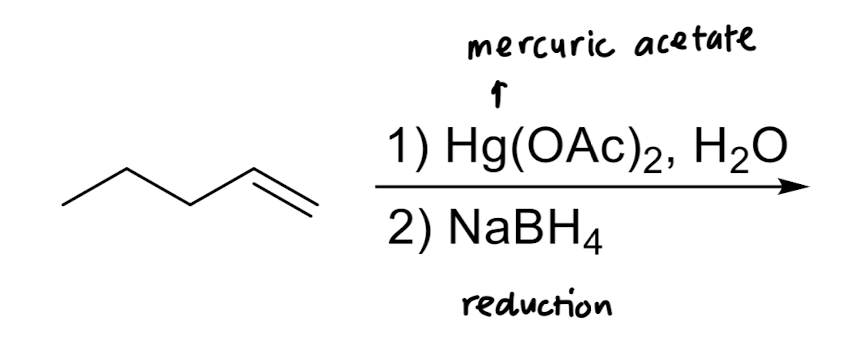
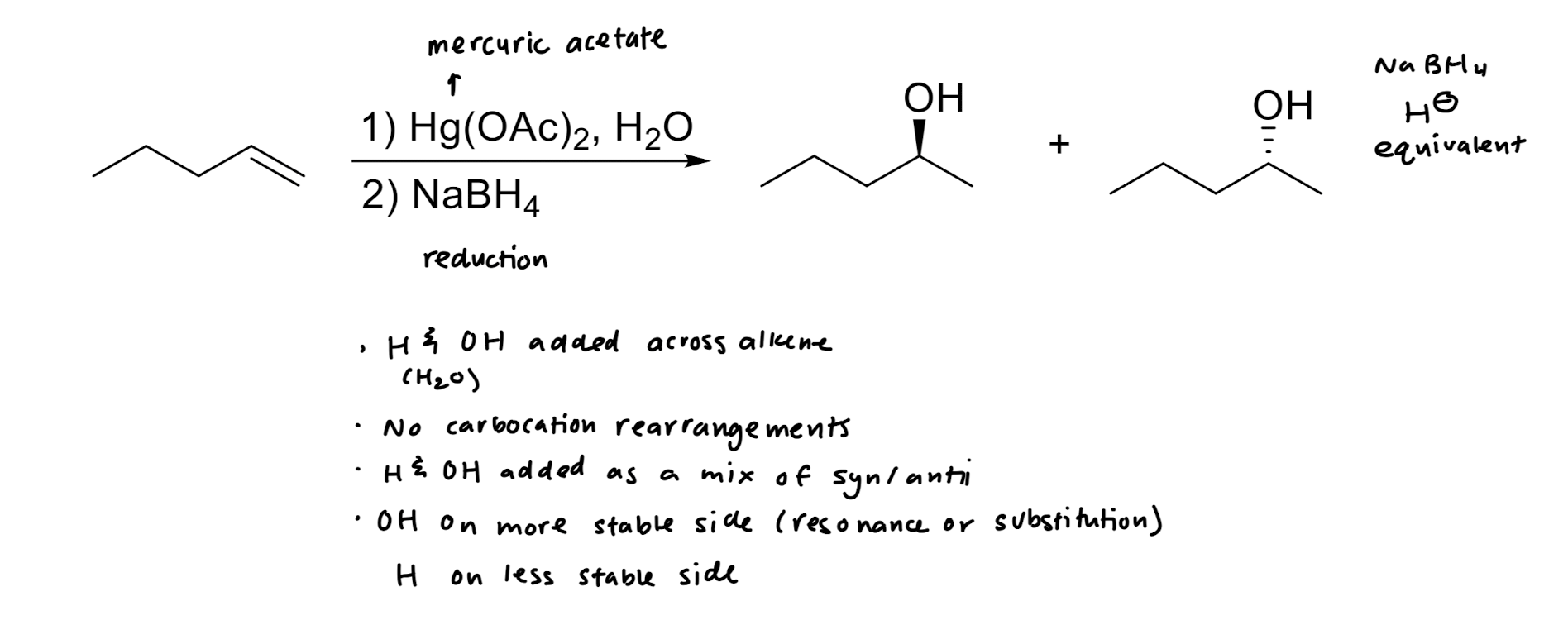
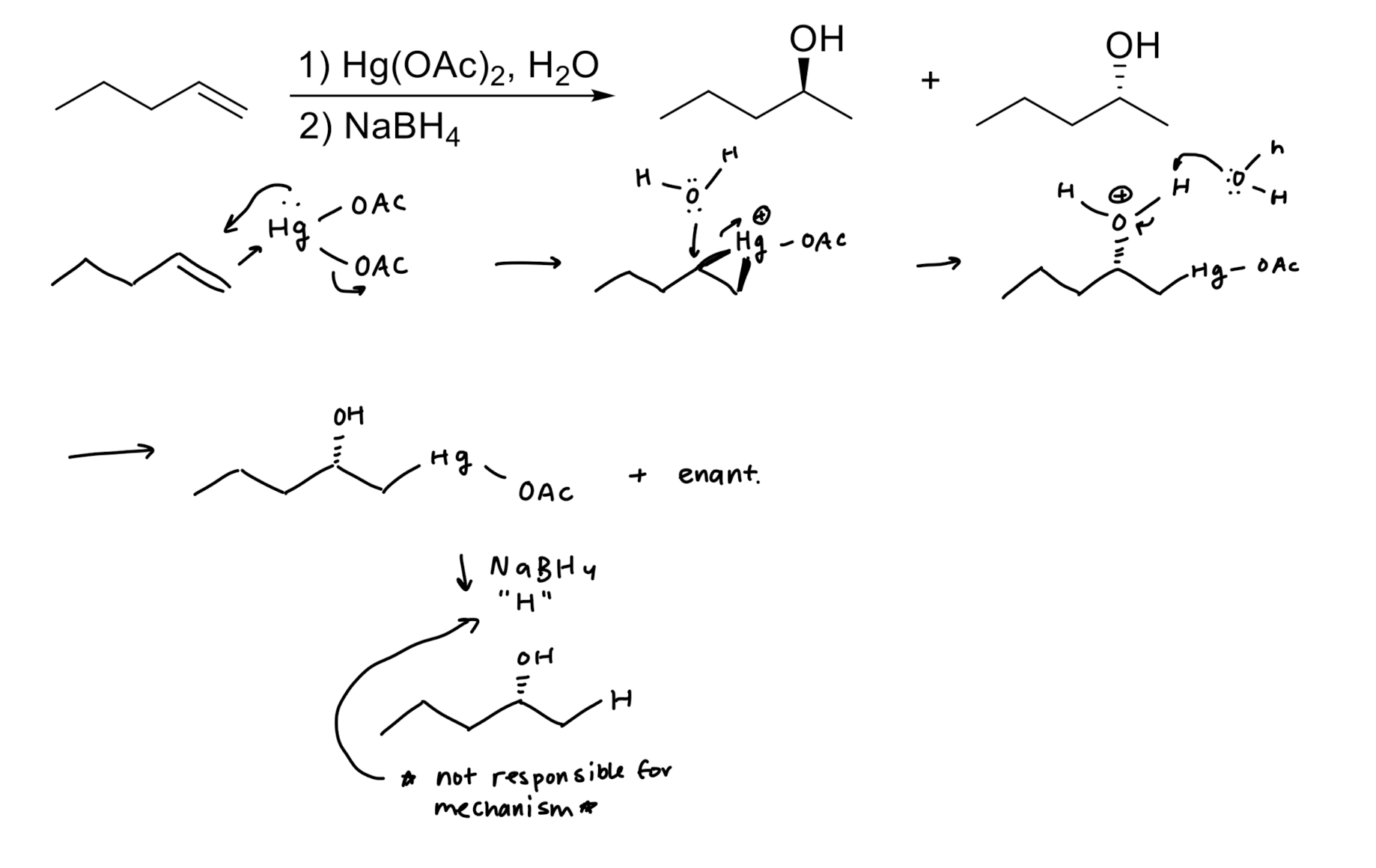
Hydration: Addition of H2O → OH/H
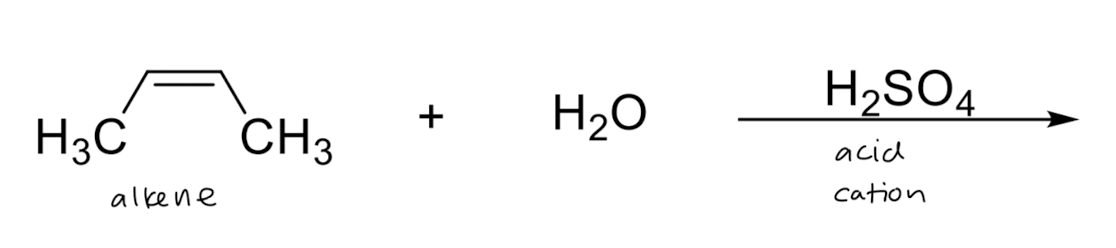
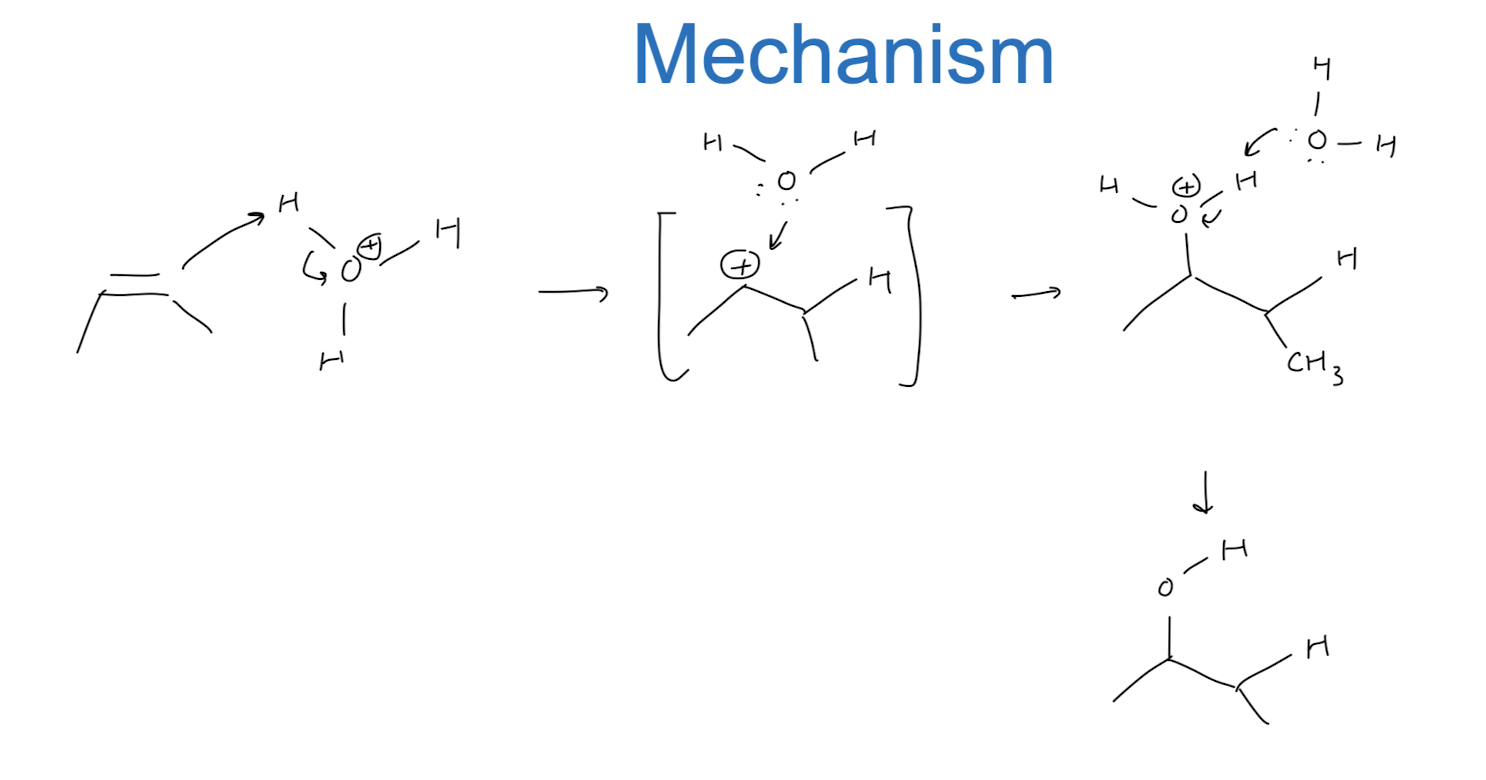
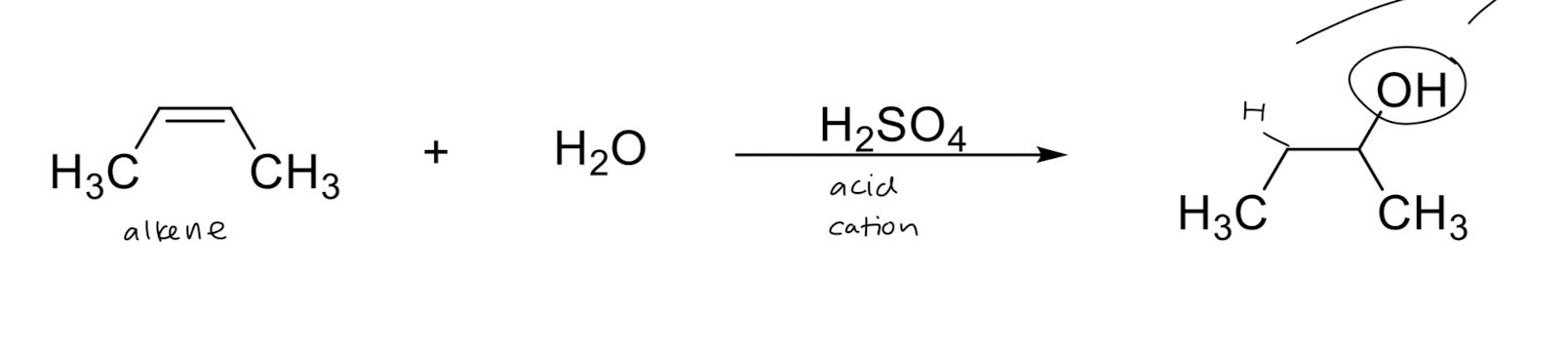
1) resonance
2) more substituted carbon
Alcohol Addition → OR/H
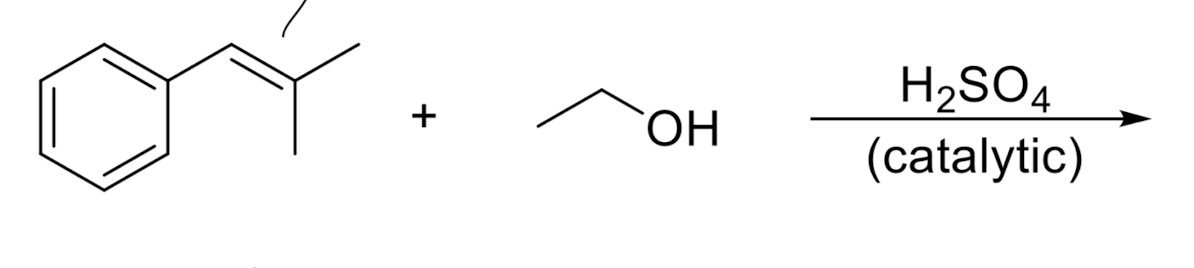
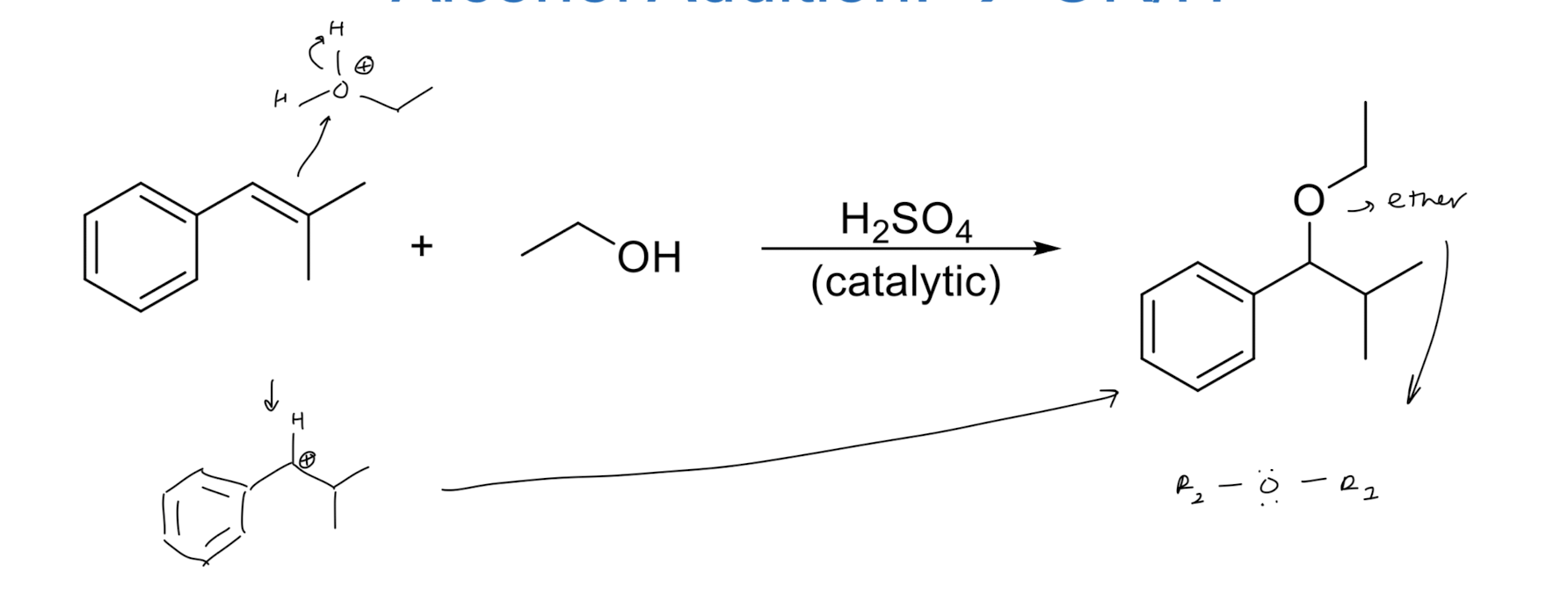
resonance stabilization
more substituted carbon
Intramolecular Reactions
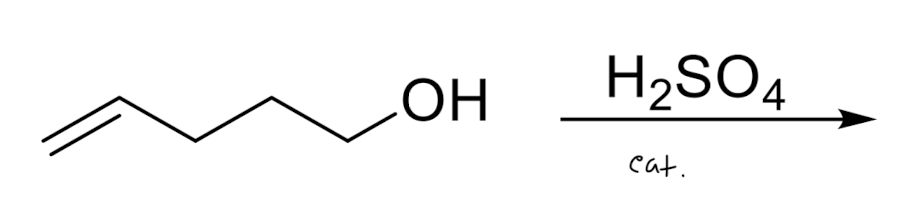
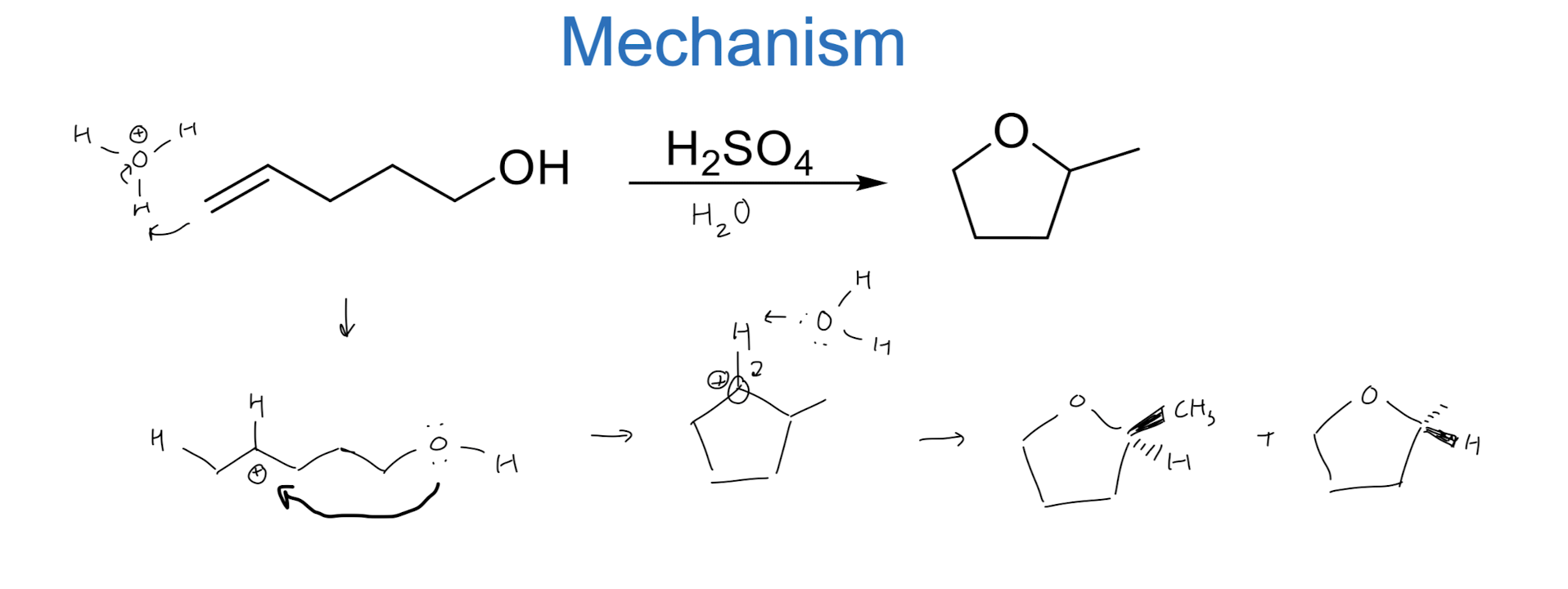
Electrophilic Addition via Three-Membered Rings
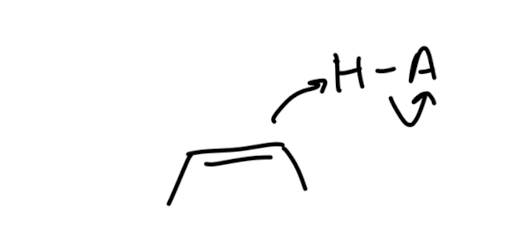
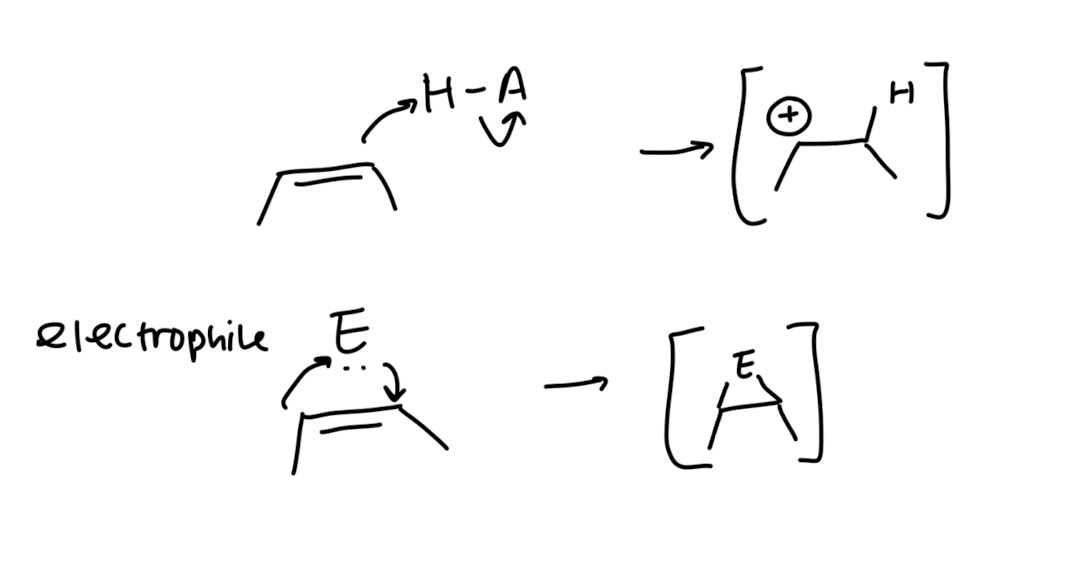
this is stereospecific
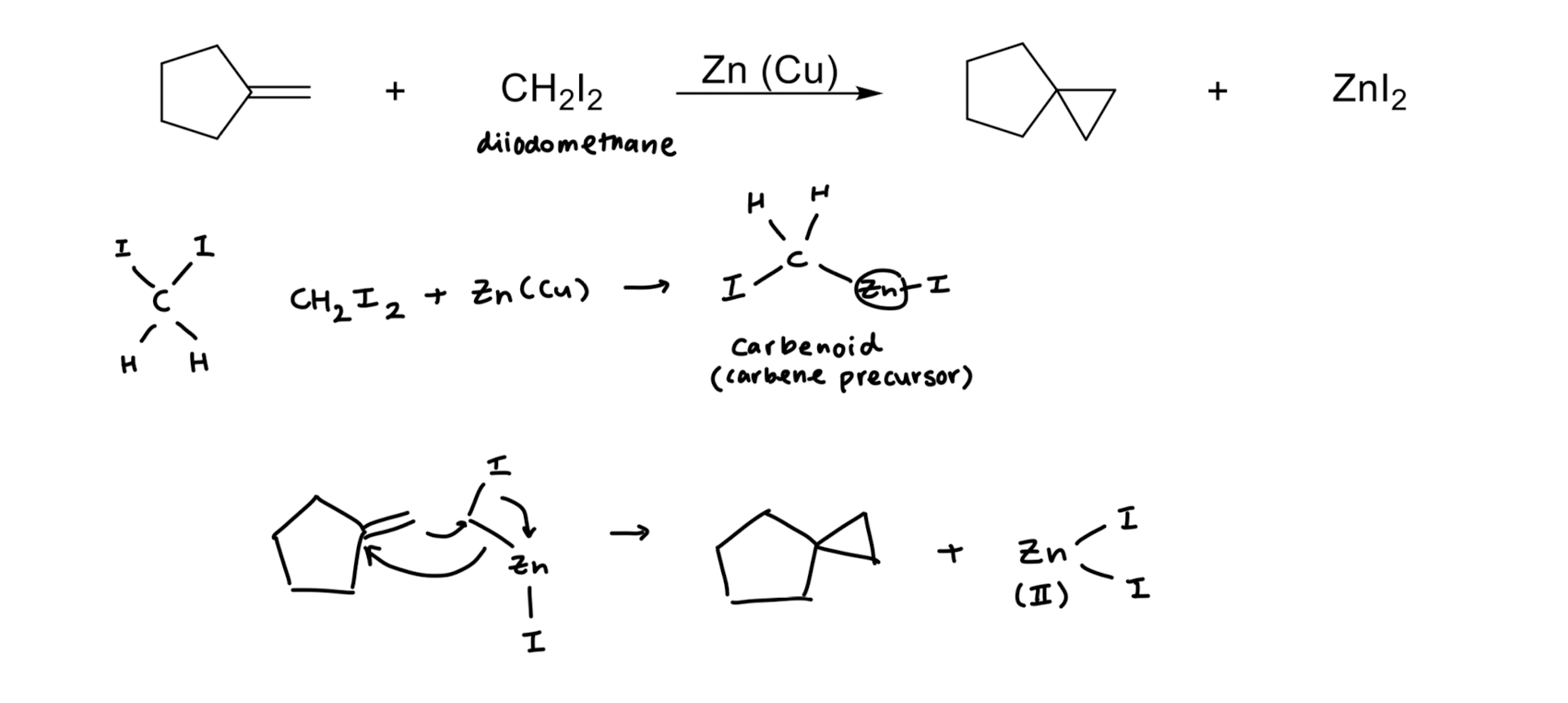
Cyclopropanation
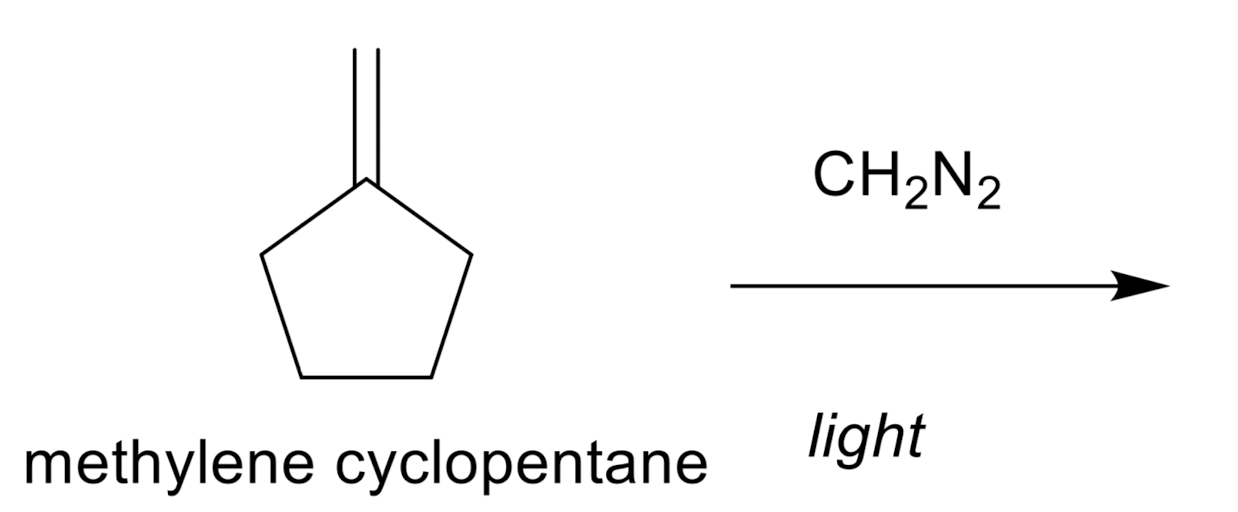
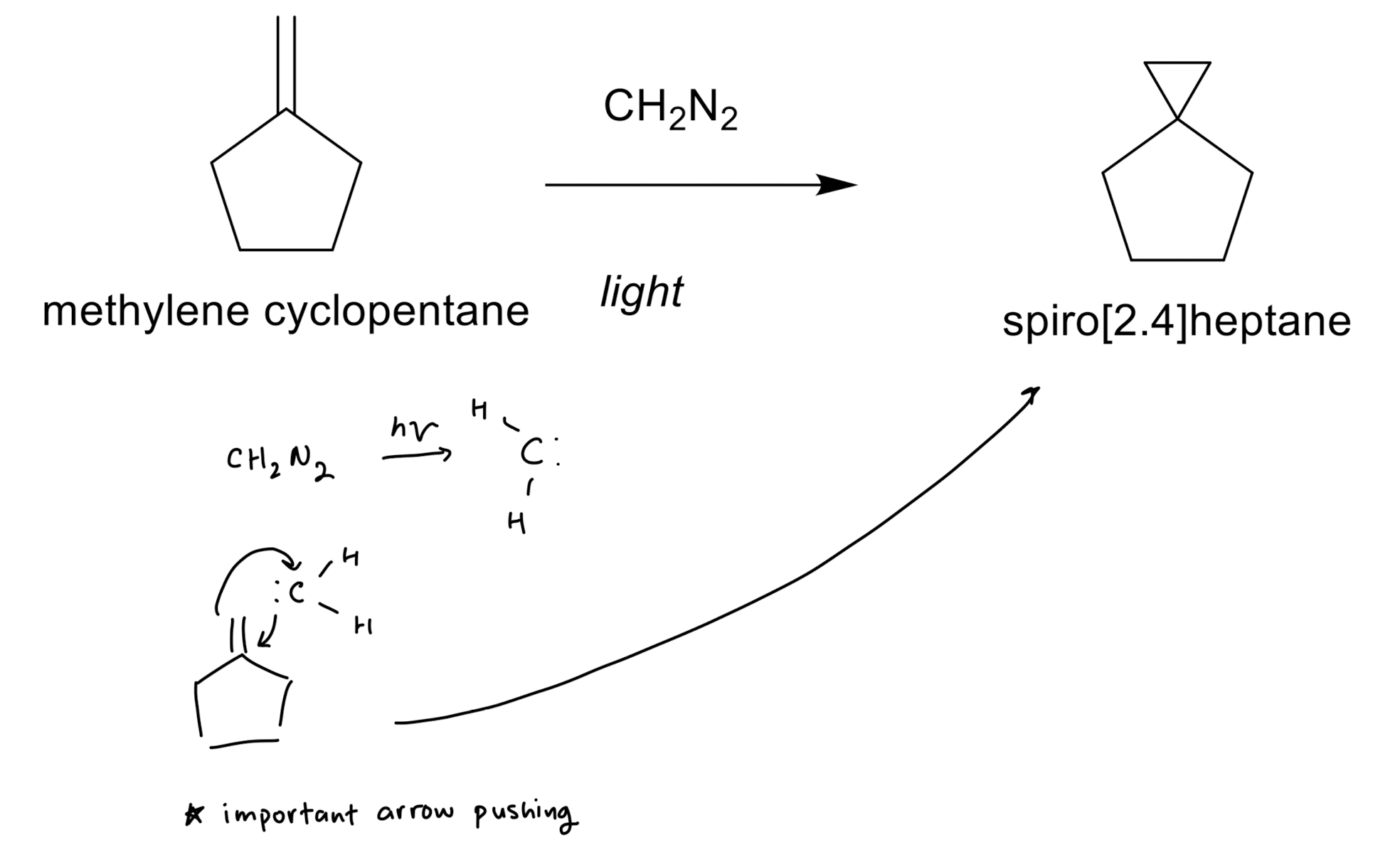
Cyclopropanation: Dichlorocarbene
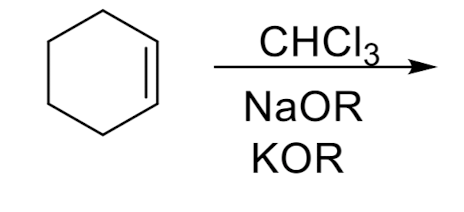
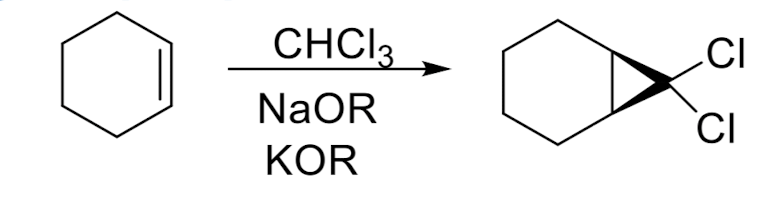
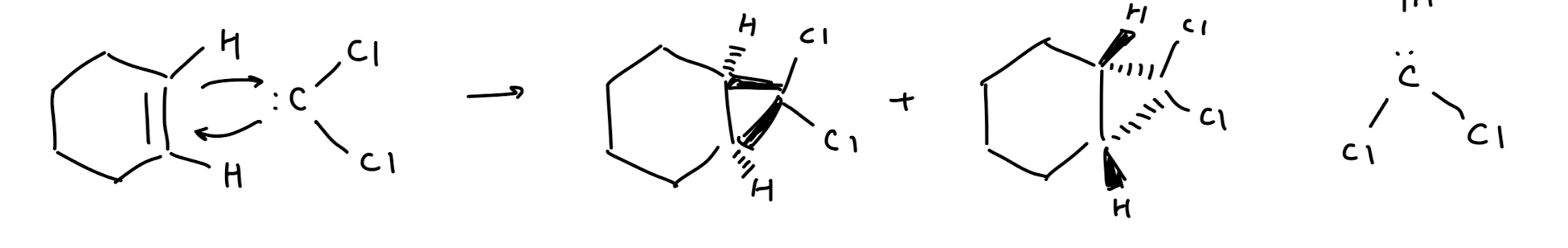
stereospecific
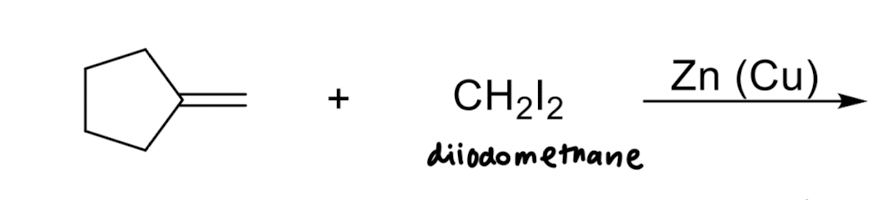
Simmons-Smith Reaction
Epoxide synthesis: peracids
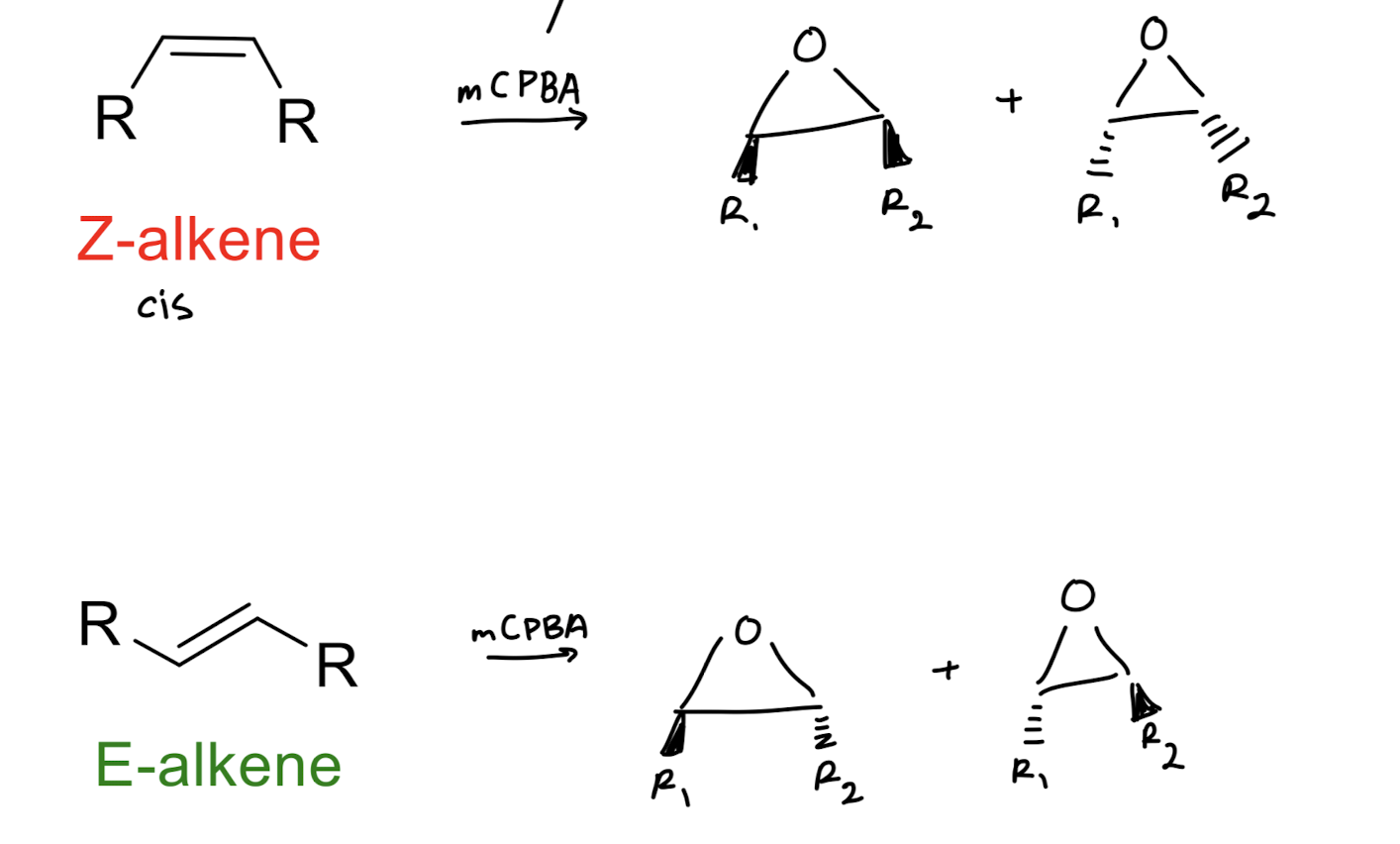
also goes for the more substituted carbons
Good Nucleophile Epoxide Opening
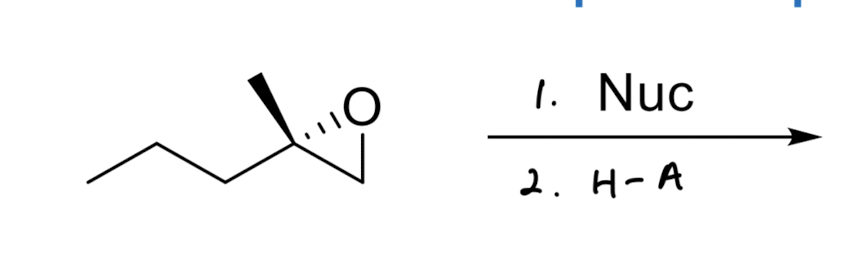
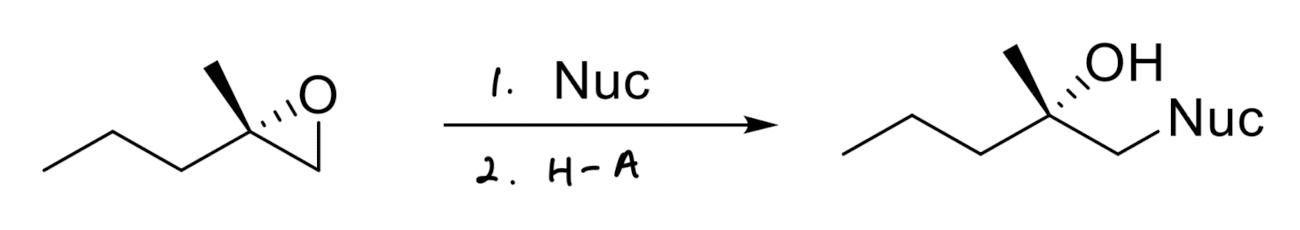
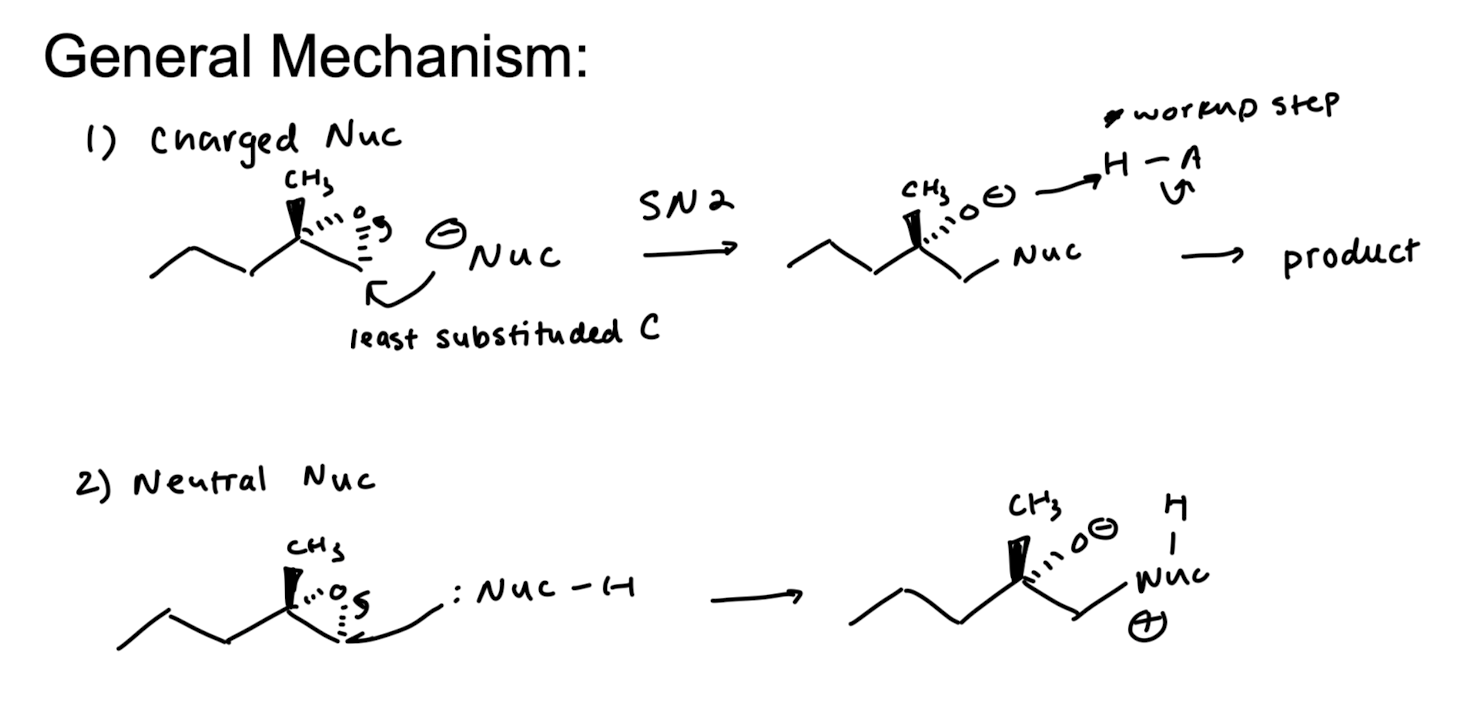
adds to the less substituted side
invert stereochemistry
Hydrides and Organometallics as Nucleophiles
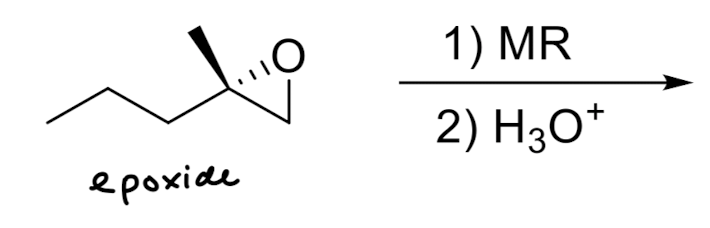
Hydride Addition
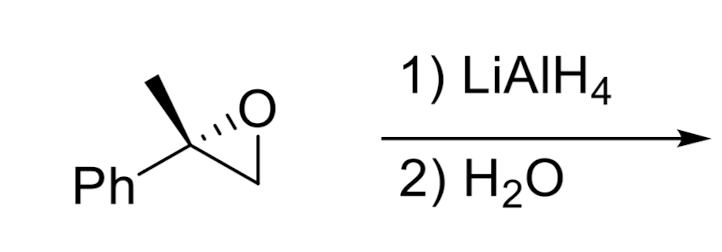
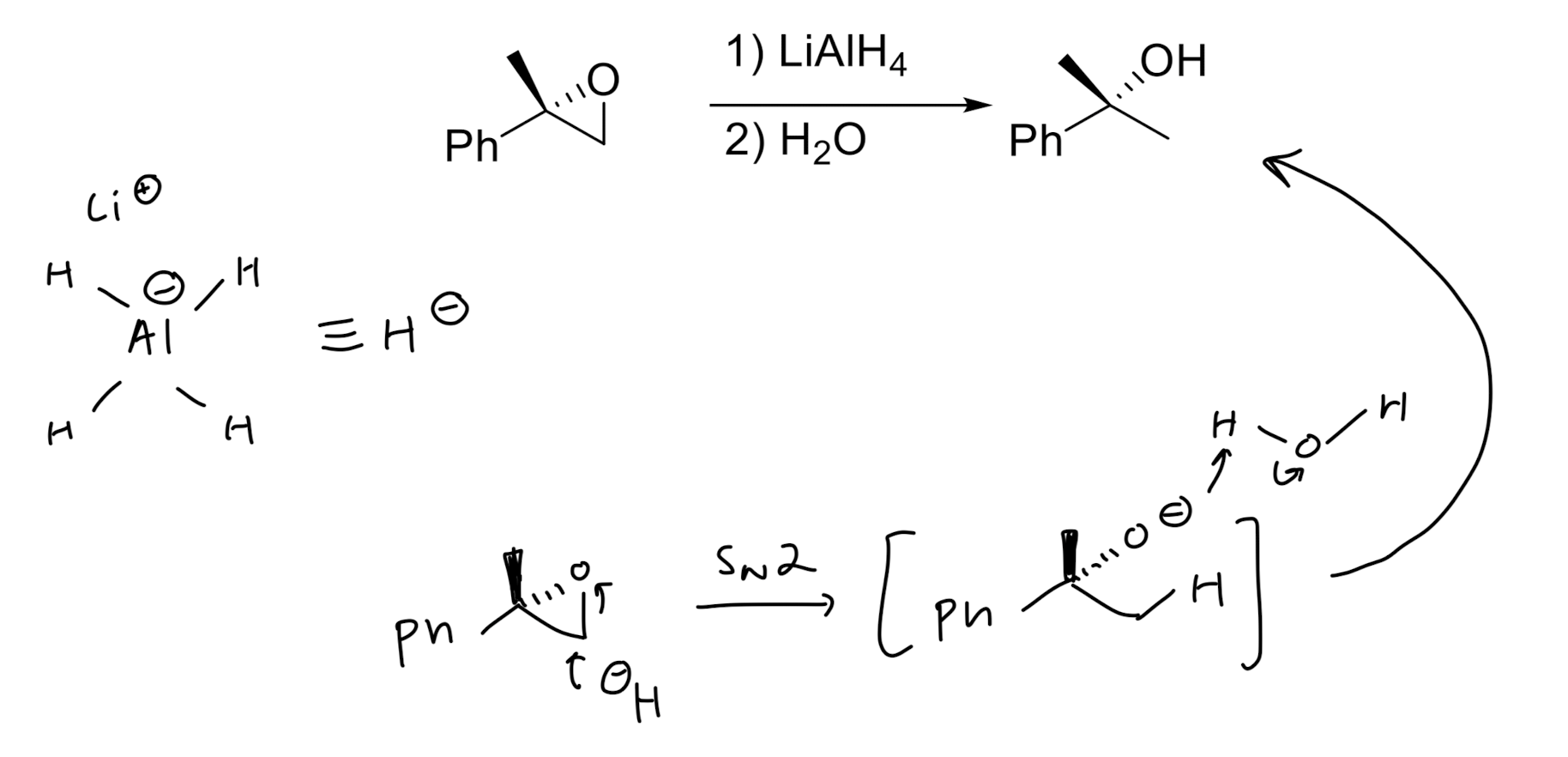
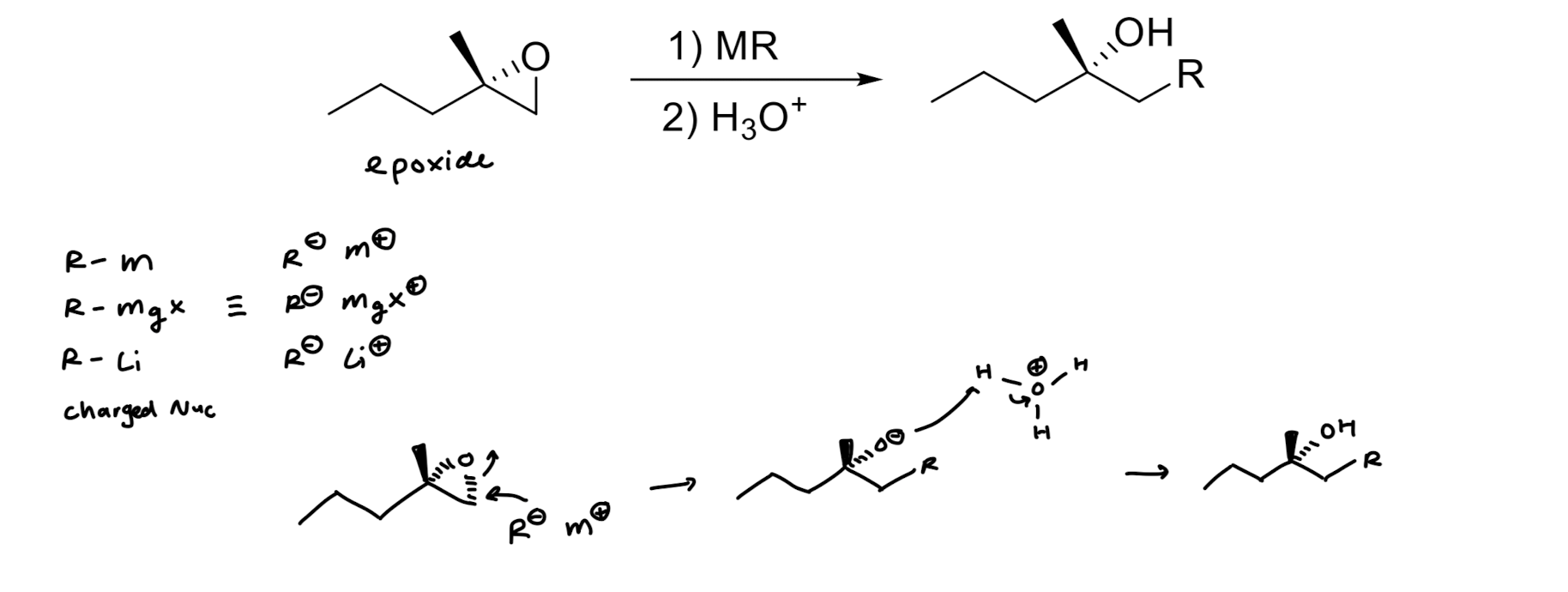
Opening of Epoxide w/ Grignard Reagents
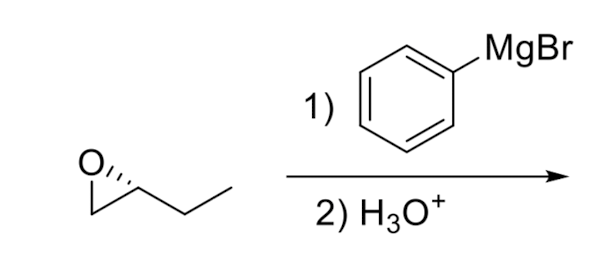
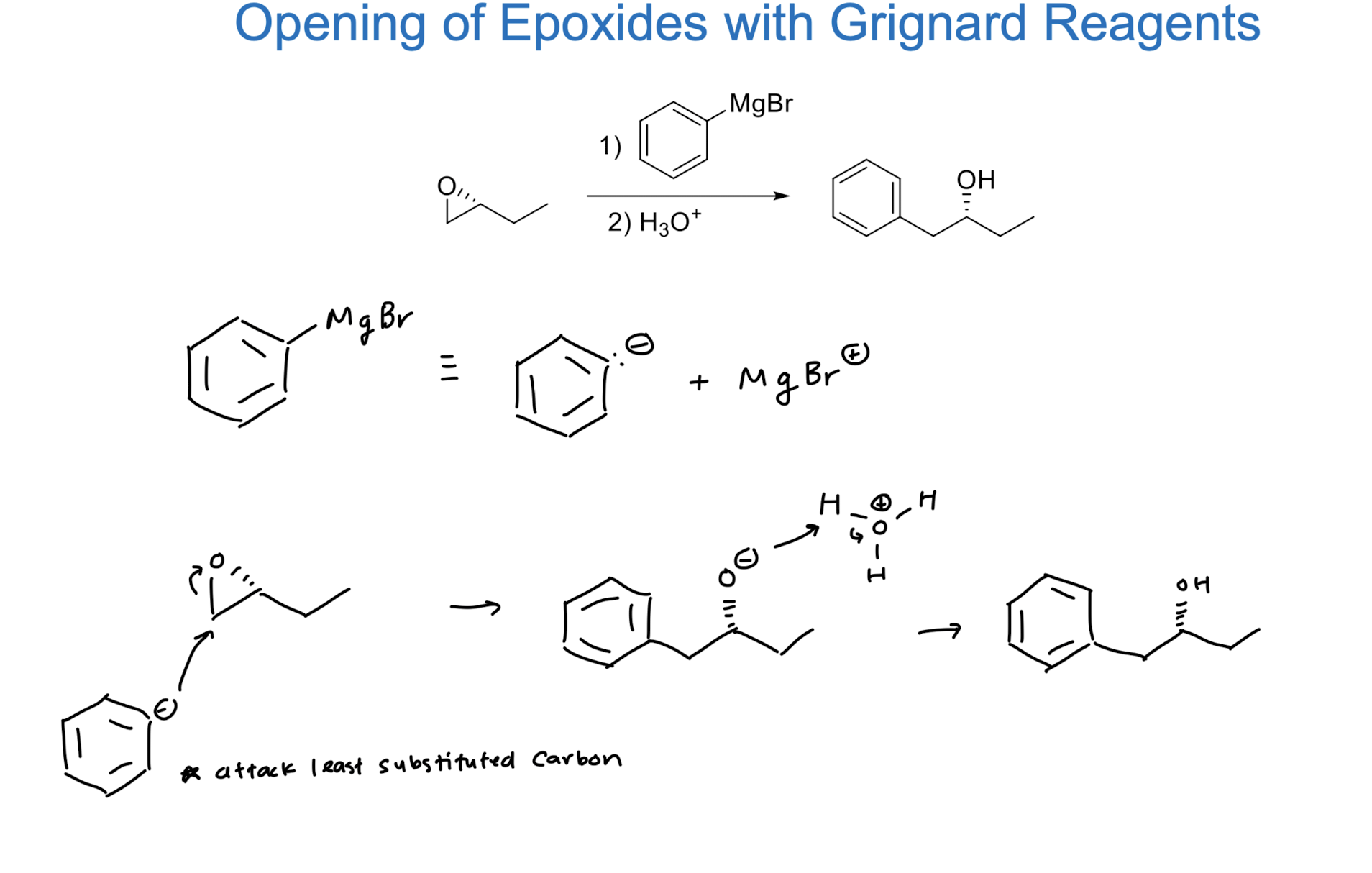
involves nucleophilic attack by Grignard reagent, leading to ring opening and formation of an alcohol.
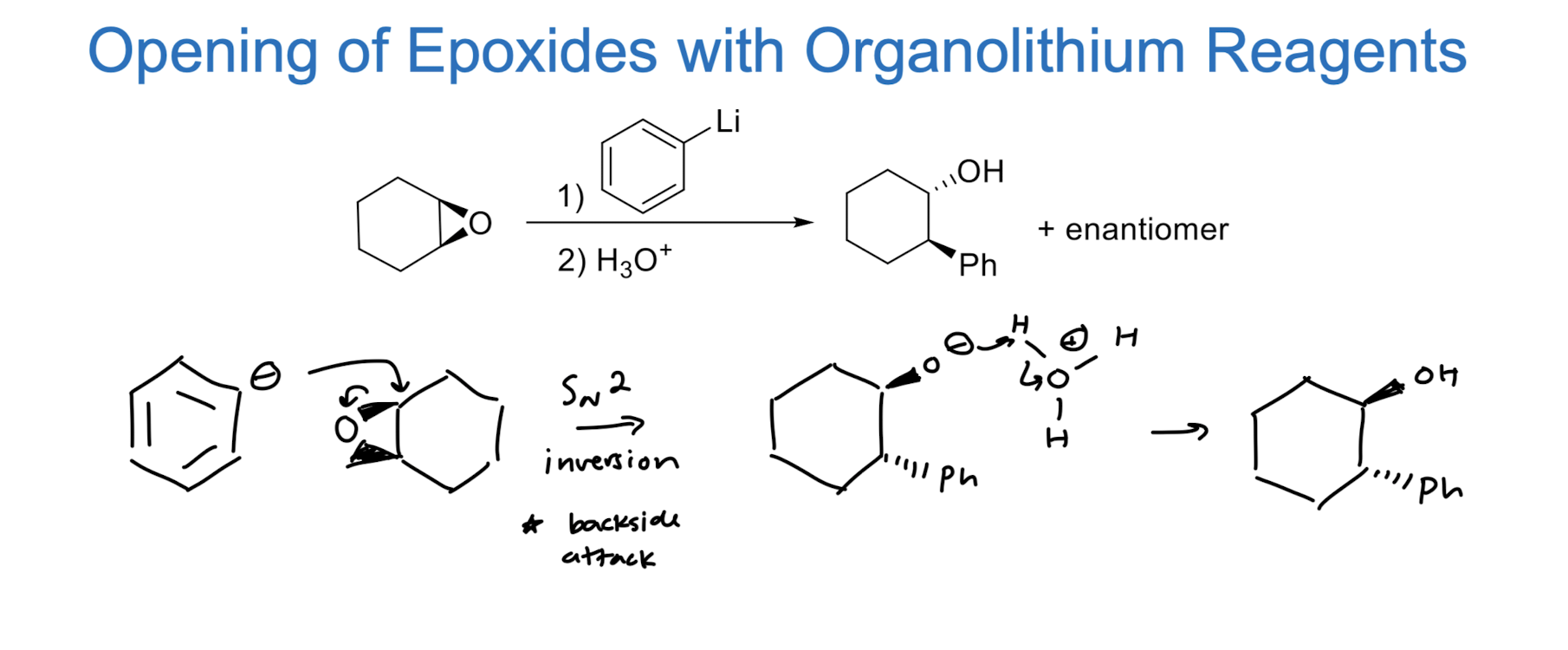
Opening of Epoxide w/ Lithium Reagents
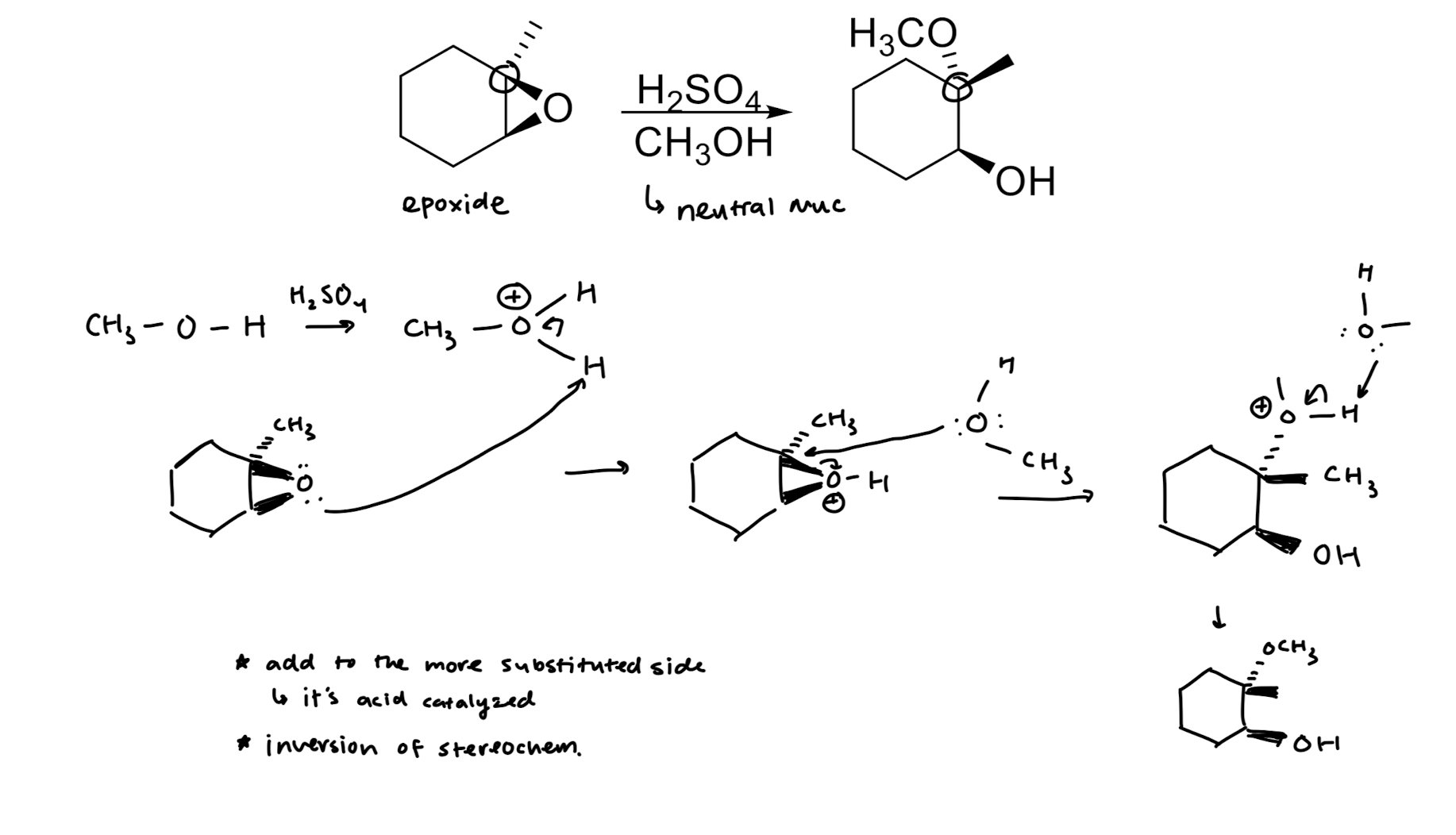
Acid Catalyzed Ring Opening
Addition of X2 to Alkenes
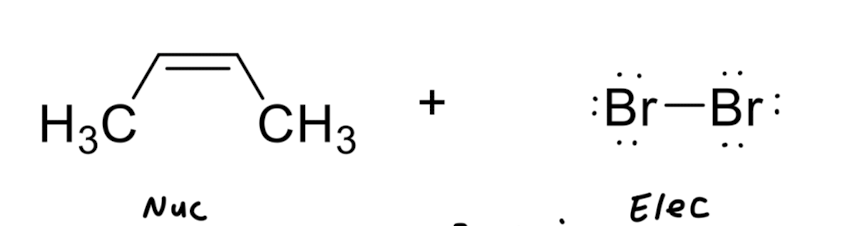
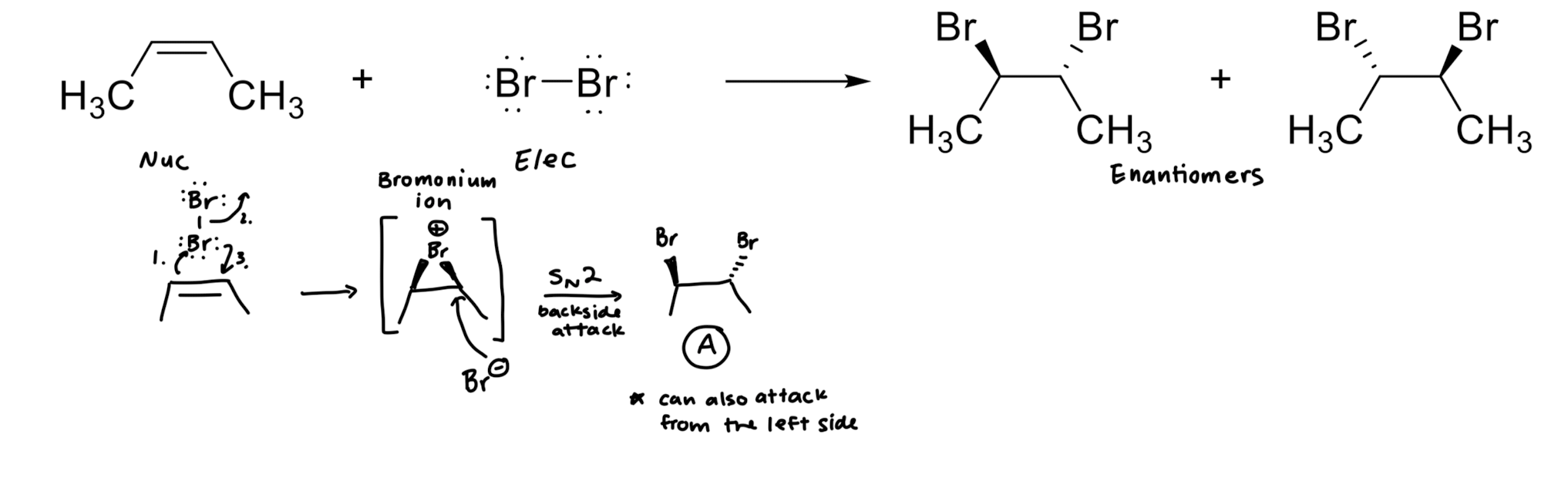
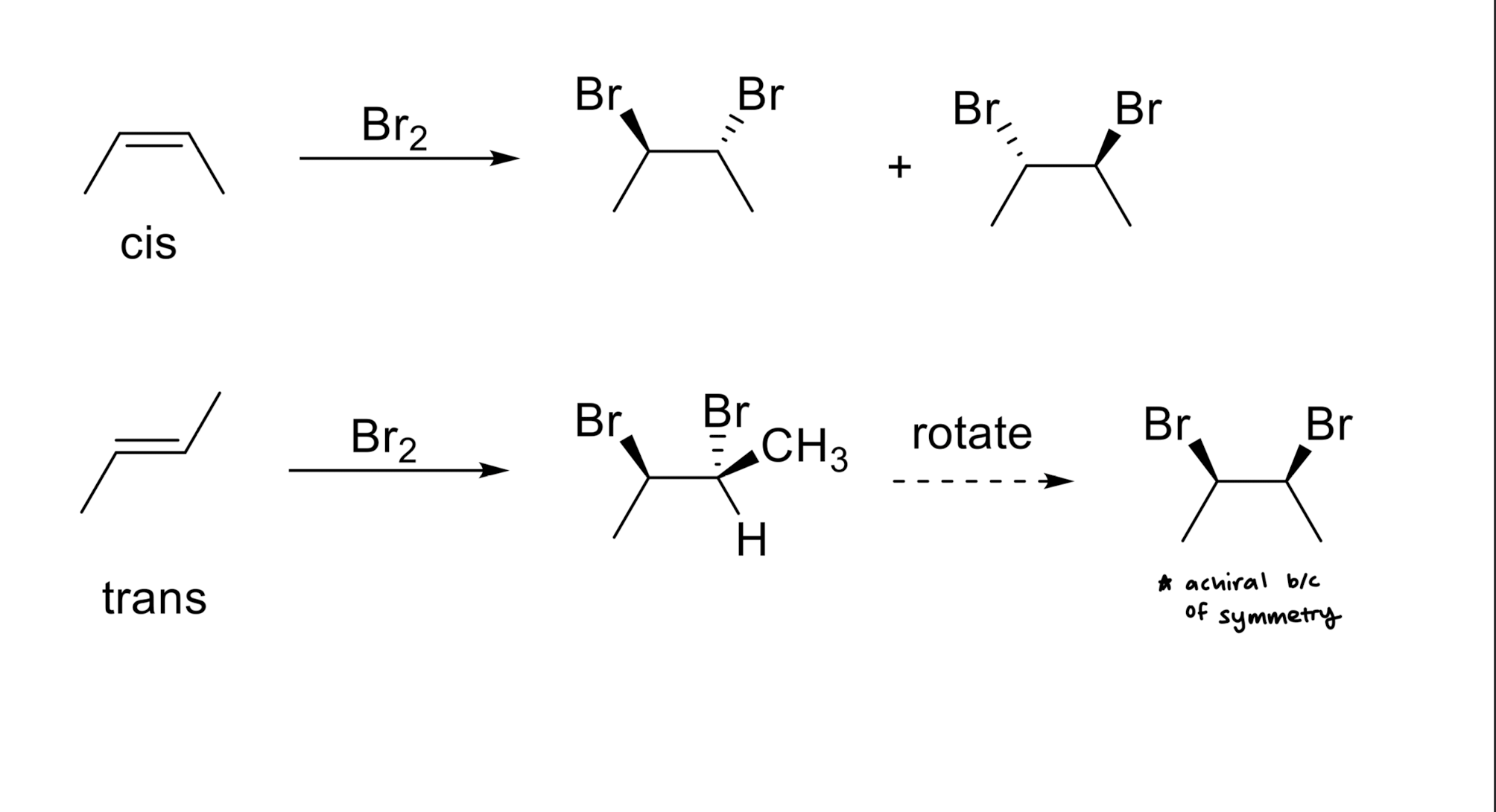
Addition of X/OH
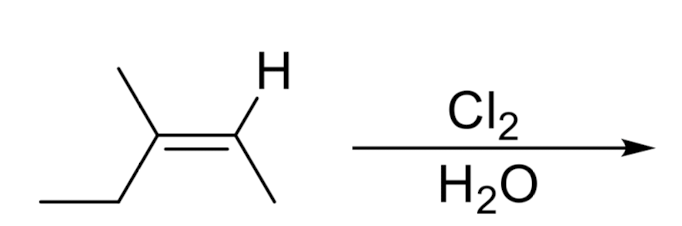
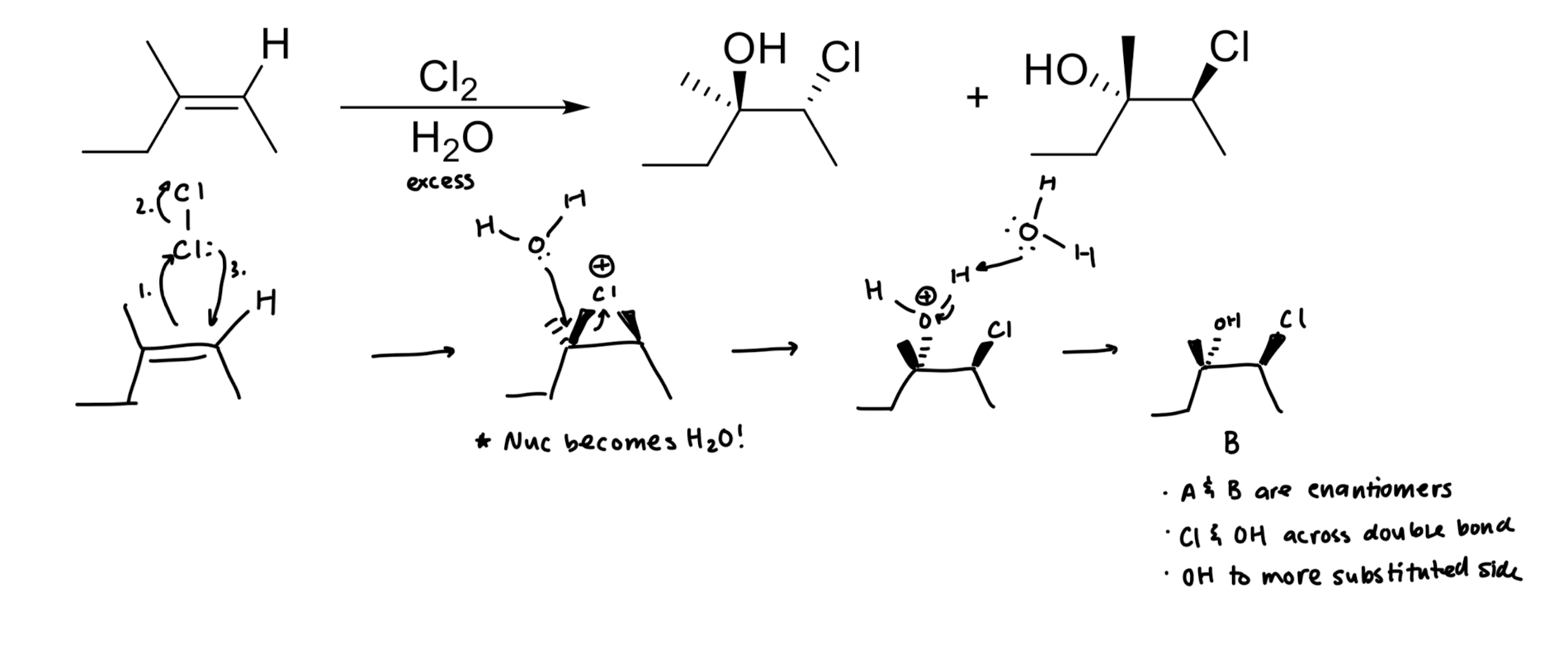
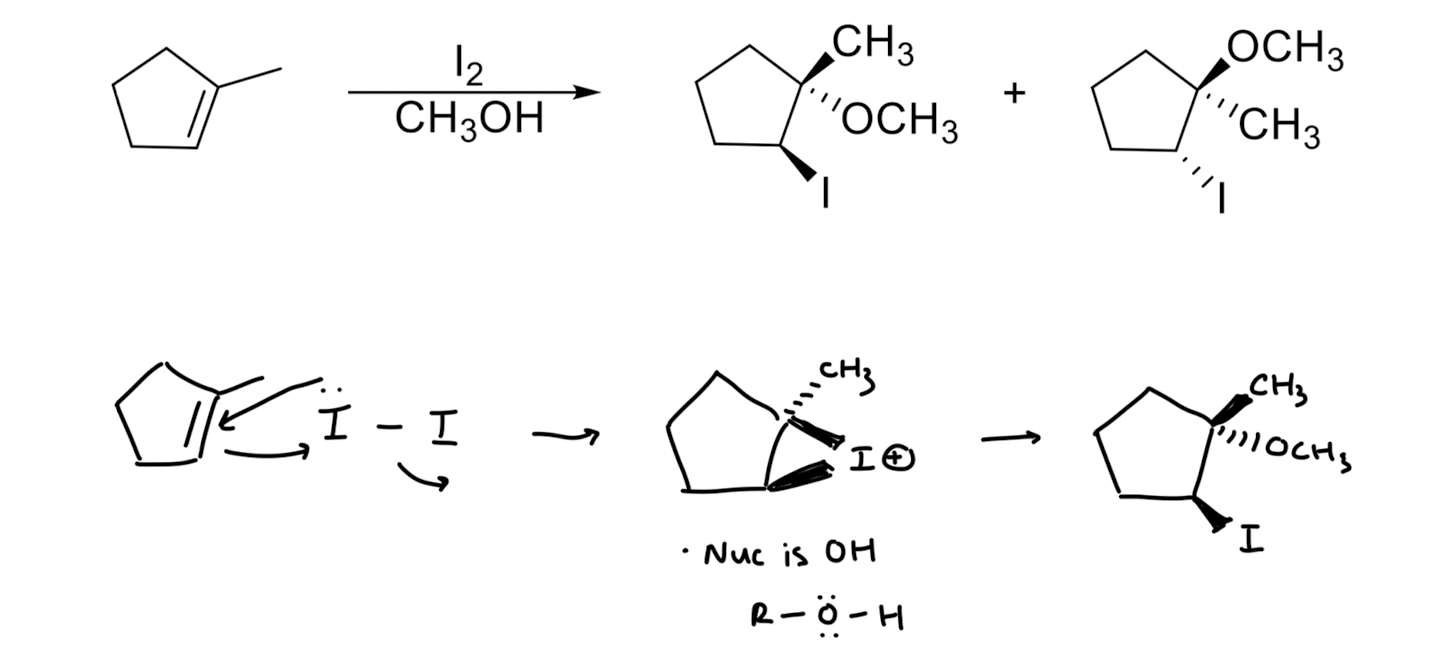
Addition of X/OR
Oxymercuration/Reduction: Addition of OH/H