2 Protein Structure
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
_____ bonds between AAs are formed by a ____________ reaction.
Amide bonds between AAs are formed by a condensation reaction.
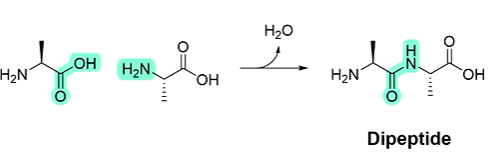
why is the central C-N bond very strong?
due to resonance
the C-N bond has partial double bond character, so only broken by very harsh chemical conditions or enzymes
outline the conformation of AAs.
Cis (Z) conformation - both substituents on the same side of the C-N bond.
trans (E) conformation - substituents on opposite sides of the C-N bond.
why are cis conformations rarer than trans conformation?
trans conformation is more stable as there is less steric chain.
describe how bonds in peptides can rotate.
peptide bonds are rigid & planar, so form a flat, straight line as shown in the diagram
bonds on either side of the C-N & C-C bonds can rotate, the R groups are alternating away from the polypeptide backbone.
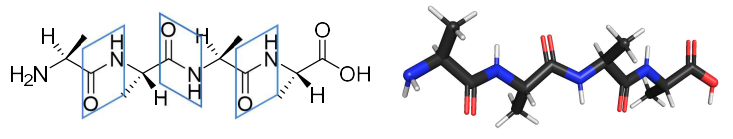
define the primary structure.
the sequence of amino acids
what is the N-terminus & C-terminus?
N-terminus - the free amino group on the end of the polypeptide.
C-terminus - the free carboxylic acid at the other end of the polypeptide
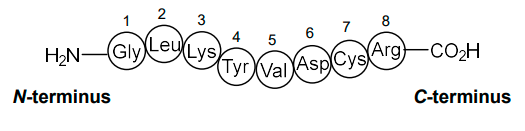
in what direction are AAs numbered?
from the N-terminus to the C-terminus (N —> C)
define secondary structure.
Local 3D structure formed by hydrogen bonding between backbone atoms
how are H bonds able to form?
due to partial ionisation of the C=O and N-H bonds.
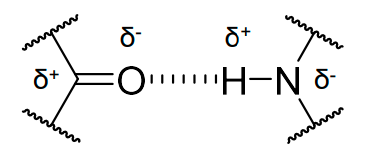
what is meant by local in the deifnition of secondary structure?
not all the AAs are involved in the secondary structure.
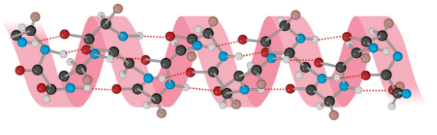
define α helices.
a single helix in which all C=O & N-H bonds of the peptide backbone are involed in H bonding
R groups point outside of the helix
define β sheets.
a secondary structure in which 2 or more peptide strand H bond to each other to form a sheet-liek sructure
unlike in α helices not every C=O & N-H bond is involved in H bonding
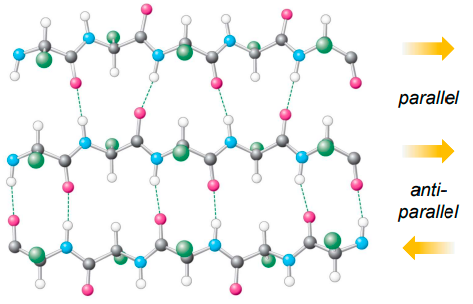
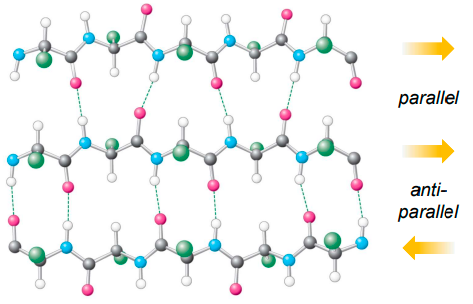
what the diff between parallel & antiparallel sheets?
parallel - peptide chains which are H bonding to each other run in same direction
antiparallel - peptde chain run in opposite direction, often linked by a turn (loop of AAs)
define tertiary structure.
Global 3D structure of a whole polypeptide chain
formed by non-covalent & covalent interactions
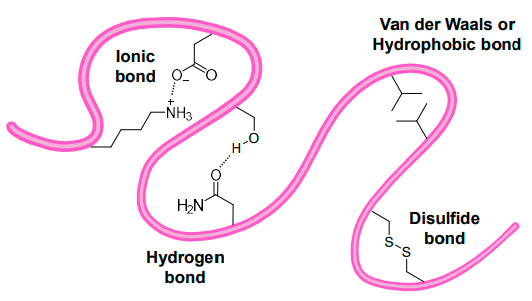
outline the non-covalent & covalent interactions.
non-covalent:
Ionic bonds
Hydrogen bonds
Hydrophobic bonds
Van der Waal’s forces
covalent:
Disulfide bonds

how do these covalent and noncovalent interactions result in proteins with diff functions?
tertary structure can produce pockets to allow entry & bonding of a protein’s substrate. e.g. in enzymes, the active site, in receptors the binding site.
globular proteins fold so hydrophilic residues are on the outside & hydrophobic residues are on the inside.
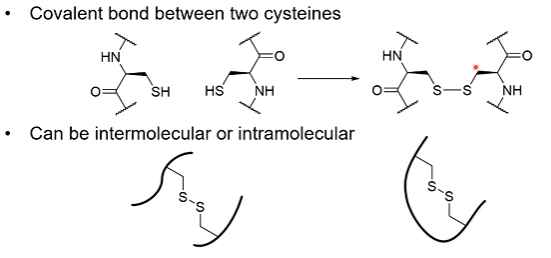
define disulfide bond.
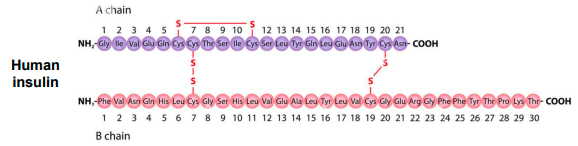
how many disulfides are there in human insulin?
there are 3
the separate polypeptides chains (A & B) are joined by 2 intermolecular disulfides
the A chain has 1 intramolecular disulfide
define quaternary structure
2 or more polypeptides assciated via non-covalent interactions
each polypeptide is called a subunit
together the protein is referred to as oligomeric
do all proteins have quarternary structure?
No