Chemistry Unit 3 Data Test Formulas
1.0(1)
Card Sorting
1/15
Last updated 11:31 PM on 8/20/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
1
New cards
equilibrium constant
the ratio of concentrations when equilibrium is reached in a reversible reaction
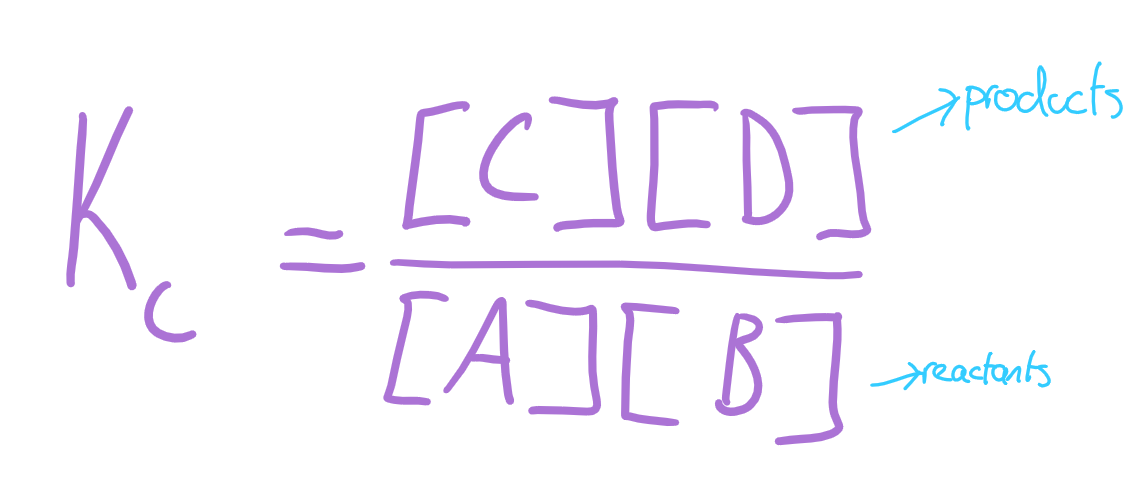
2
New cards
pH
the logarithm of the reciprocal of hydrogen-ion concentration in gram atoms per liter
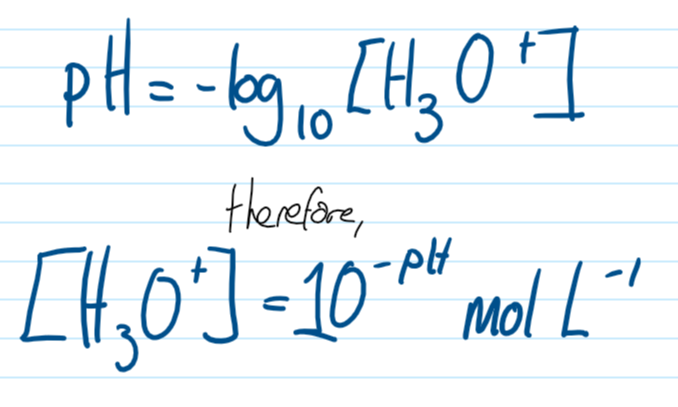
3
New cards
pKw
the negative log of the water ion product
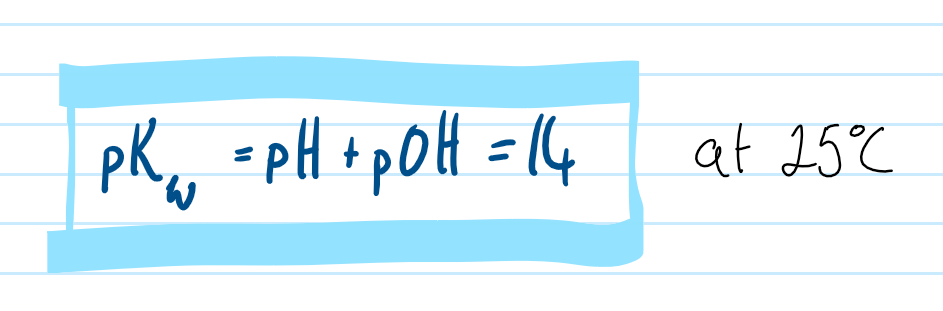
4
New cards
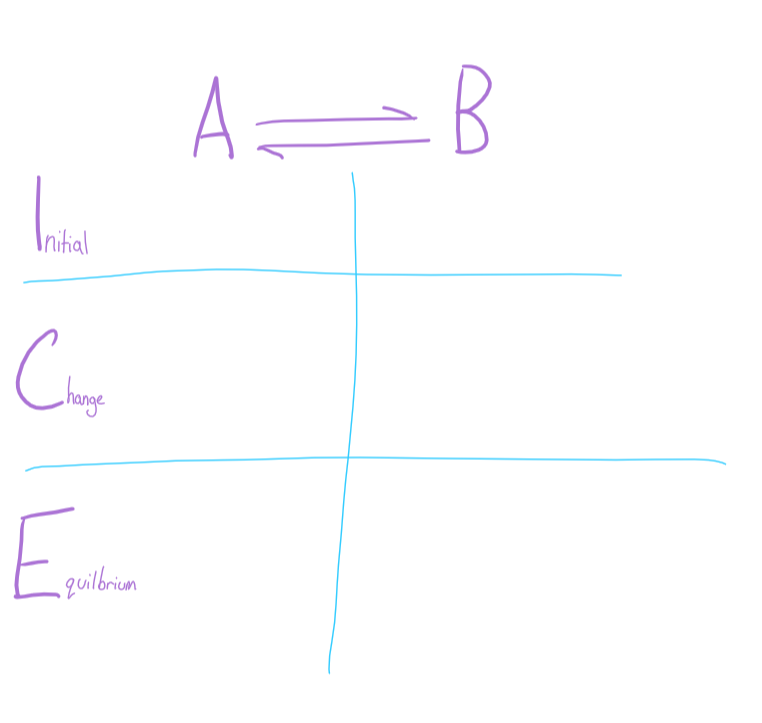
ICE Table
tabular system of keeping track of changing concentrations in an equilibrium reaction
5
New cards
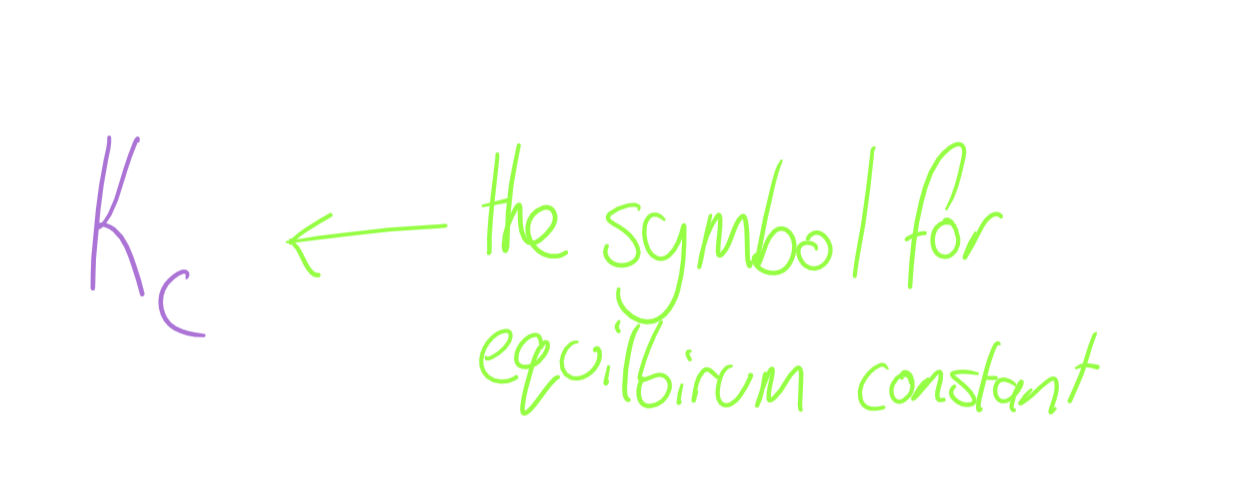
Equilibrium Constant Symbol
6
New cards
equilibrium expression
"the equation without any numbers'

7
New cards
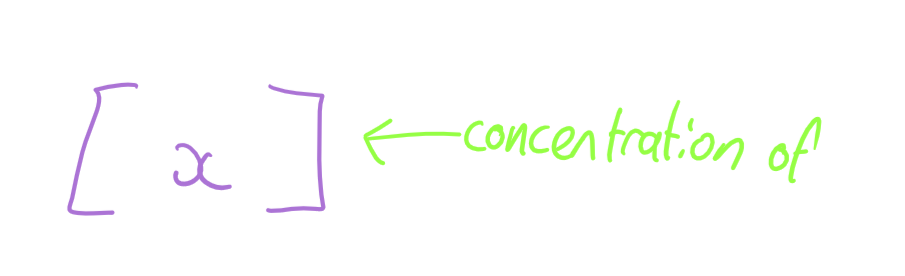
square brackets
concentration of
8
New cards
diprotic
has two equivalence points
9
New cards
Kb formula
10
New cards
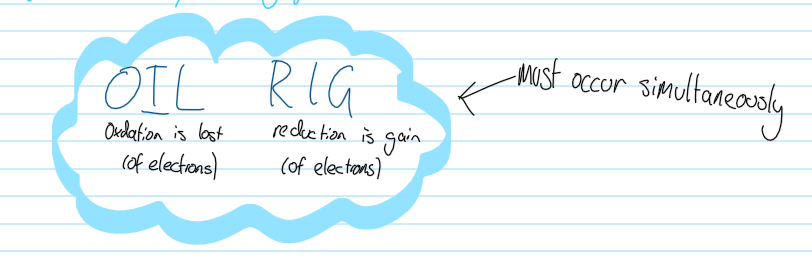
Redox Reaction acronym
Oil Rig
11
New cards
Oxidation Rule 1
* Any substance in its __**elemental state**__ is given the oxidation number __**zero.**__
* They have neutral charge
* Examples O2, H2, Fe, P4
* They have neutral charge
* Examples O2, H2, Fe, P4
12
New cards
Oxidation Rule 2
* The oxidation number for a __**monatomic**__ ion is simply the __**charge for that ion**__
* Examples, Na+ = +1, K+ = + 1, Cl- =-1
* Examples, Na+ = +1, K+ = + 1, Cl- =-1
13
New cards
Oxidation Rule 3
In compounds...
* __**H is always +1**__ (except for metal hydrides: H=-1)
* __**O is always -2**__ (except peroxides where it is -1, or when it forms a compound with Fluorine)
* Halogens - F, Cl, Br and I - are -1 (except where they combine with a more electronegative element)
* __**H is always +1**__ (except for metal hydrides: H=-1)
* __**O is always -2**__ (except peroxides where it is -1, or when it forms a compound with Fluorine)
* Halogens - F, Cl, Br and I - are -1 (except where they combine with a more electronegative element)
14
New cards
Oxidation Rule 4
* The sum of the oxidation numbers in all atoms of a __**neutral**__ compound __**must be 0**__
15
New cards
Oxidation Rule 5
* The sum of the oxidation numbers of all the atoms in a __**polyatomic**__ ion is __**equal to the charge of the ion**__
16
New cards
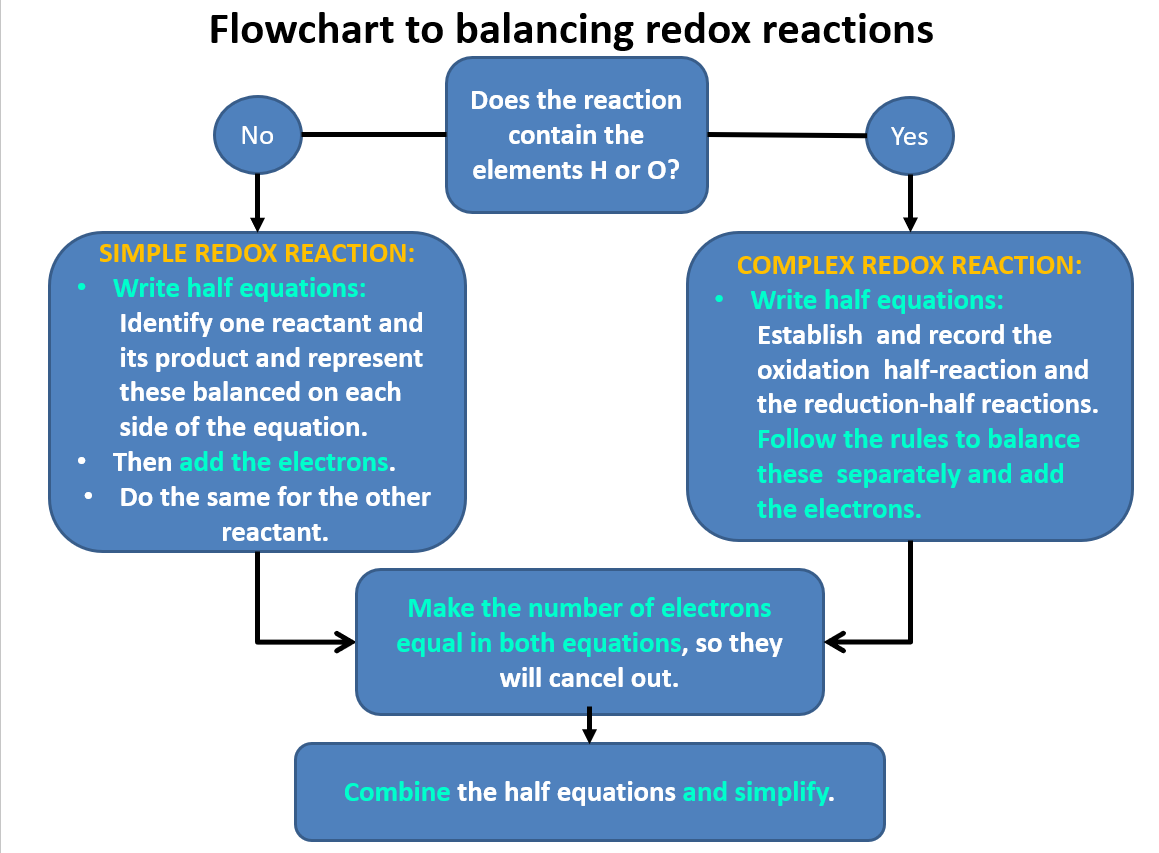
Balancing Redox Reactions