Properties of alcohols
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
General formula
Cn H2n+1 OH
Nomenclature
- Ol at the end
2 hydroxyl groups = diol
3 hydroxyl groups = triol
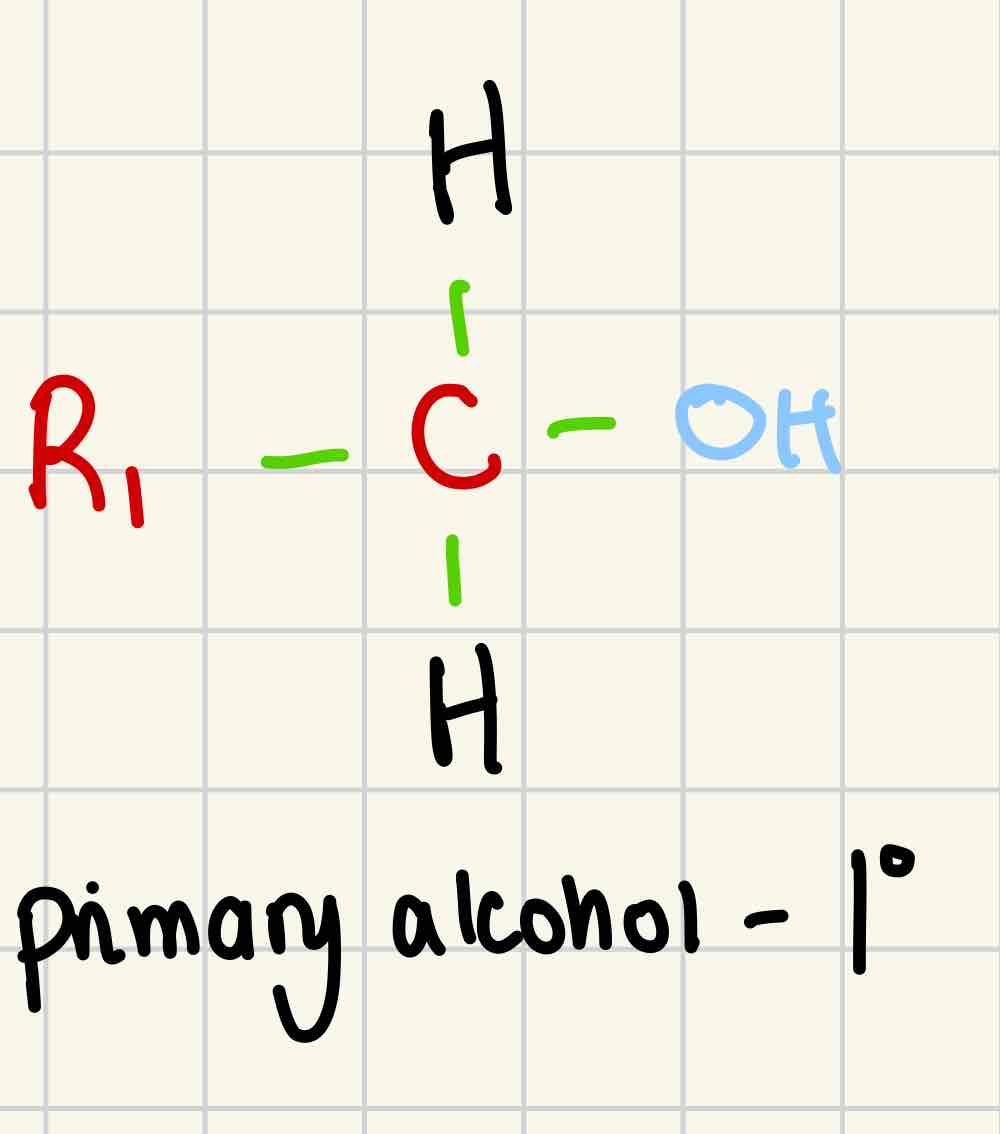
Primary alcohol
1º
- OH is attached to a carbon with one alkyl group attached
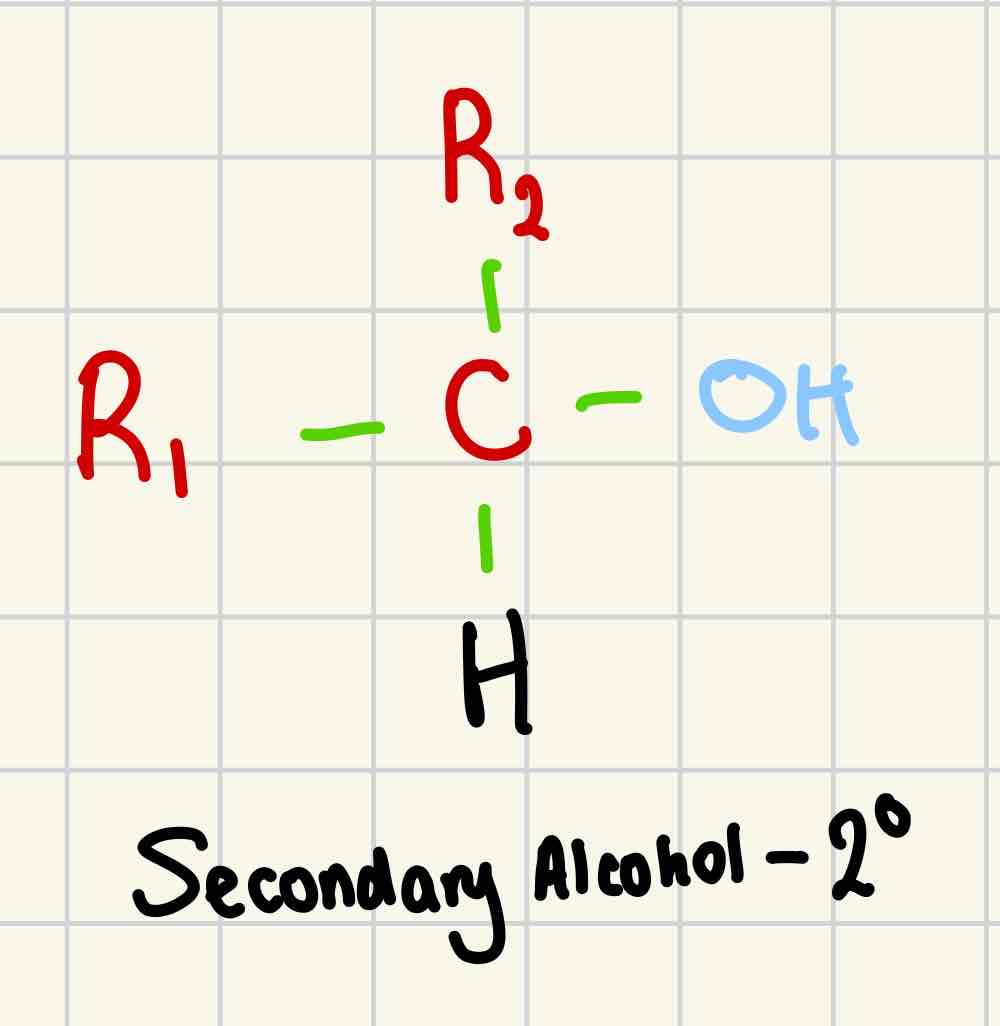
Secondary alcohol
2º
- OH group is attached to a carbon with 2 alkyl groups attached
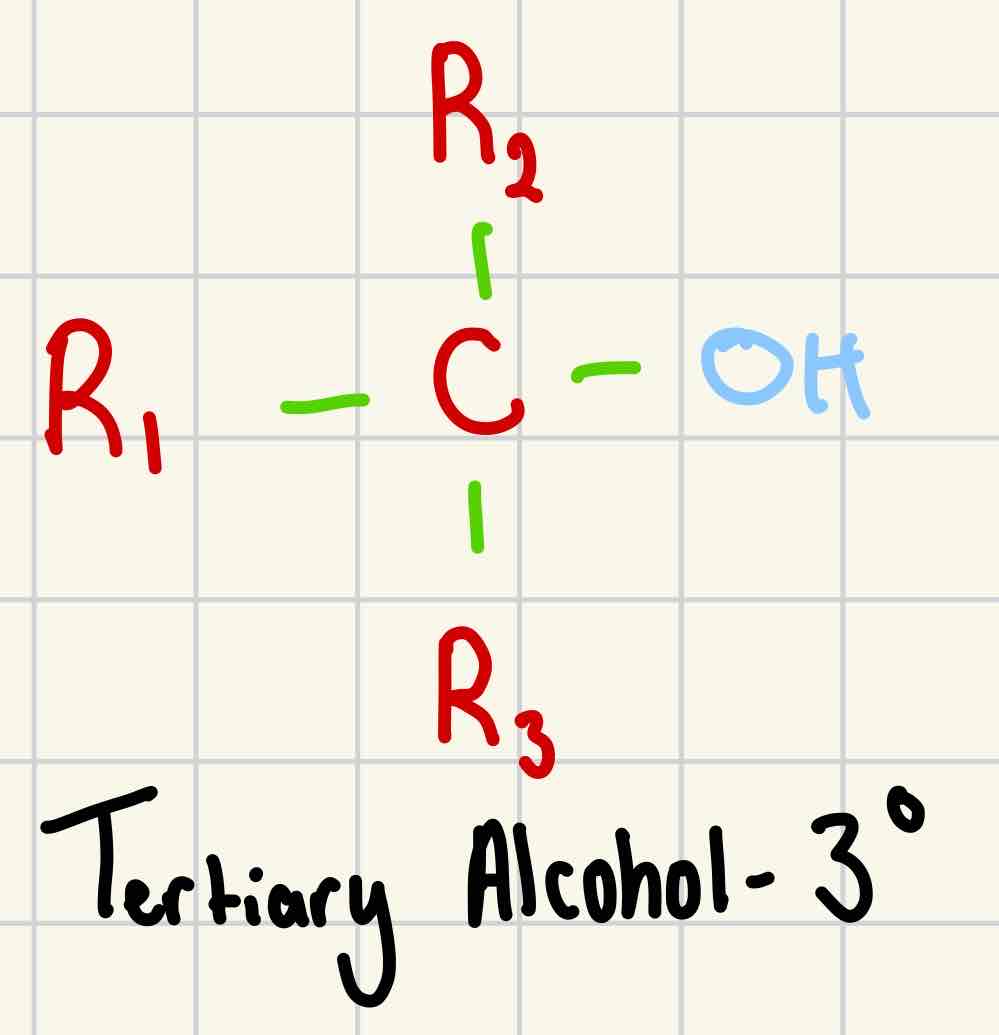
Tertiary alcohol
3°
-OH group is attached to a carbon with 3 alkyl groups attached
Physical properties of alcohols
Polar → has electronegative hydroxyl group (pulls electrons in c-oh bond away from carbon atom )
-OH group is also polar → has δ- on oxygen δ+ on hydrogen = hydrogen bonds can form
Low volatility due to hydrogen bonds (strong intermolecular forms) so they don't evaporate easily into gas
Trend in hydrogen bonding
Small alcohols → hydrogen bonding allows for solubility in water
Larger alcohol → most of molecule is non-polar = decreased solubility in water