Structure of Matter
0.0(0)
Card Sorting
1/32
Earn XP
Last updated 5:39 AM on 1/11/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
1
New cards
What are the 4 elements that make up 97% of all living matter?
Carbon, Oxygen, Hydrogen, Nitrogen
2
New cards
What percentages make trace elements?
less than .01 %
3
New cards
What are some elements found in trace elements?
boron, chromium, cobalt, copper, fluorine, iodine, iron, manganese, molybdenum, selenium, silicon, tin, vanadium, zinc (Buy Childish Clothing Cuz Father Is Invincible My Mom Sells Secret Trees Very Zoo)
4
New cards
Where do we get elements?
Diets and supplements
5
New cards
Fill in the blank: _______, organelle, cell, tissue, organ, organ system, organism
compound
6
New cards
Fill in the blank: compound, ___________, cell, tissue, organ, organ system, organism
organelle
7
New cards
Fill in the blank: compound, organelle,___, tissue, organ, organ system, organism
cell
8
New cards
Fill in the blank: compound, organelle, cell, ____, organ, organ system, organism
tissue
9
New cards
Fill in the blank: compound, organelle, cell, tissue, _________, organ system, organism
organ
10
New cards
Fill in the blank: compound, organelle, cell, tissue, organ,______, organism
organ system
11
New cards
Fill in the blank: compound, organelle, cell, tissue, organ, organ system, _______
organism
12
New cards
What do organic compounds usually contain?
carbon
13
New cards
What is matter?
anything that takes up space
14
New cards
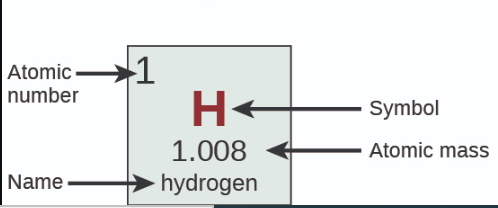
Atomic Number = ?
number of protons
15
New cards
Electron Number = ?
proton number if atom is stable
16
New cards
Mass Number is what?
atomic weight rounded to the nearest whole number
17
New cards
Mass number = ?
atomic weight rounded to the nearest whole number
18
New cards
_________ - atomic number = number of neutrons (MAN)
Mass number
19
New cards
Mass number -________ = number of neutrons (MAN)
atomic number
20
New cards
Mass number - atomic number = ___________ (MAN)
number of neutrons
21
New cards
What are Isotope?
atoms with varying number of neutrons
22
New cards
What can Isotopes be used for?
Radioactive isotopes are used in biology as tracers and as for medical treatments
23
New cards
What are Ions?
atoms that loses or gains electrons
24
New cards
Has Positive ions gained or lost electrons?
lost
25
New cards
How are Positive Ions written?
\
Na^+
Na^+
26
New cards
What do Positive Ions represent?
sodium atom that lost 1 electron
27
New cards
Has Negative ions gained or lost electrons?
gained
28
New cards
How are negative ions written?
O^2-
29
New cards
What do negative ions represent?
oxygen atoms that has gained 2 electrons
30
New cards
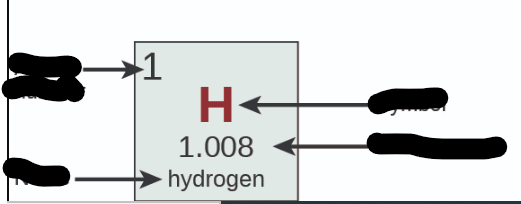
Where is Atomic Number?
31
New cards
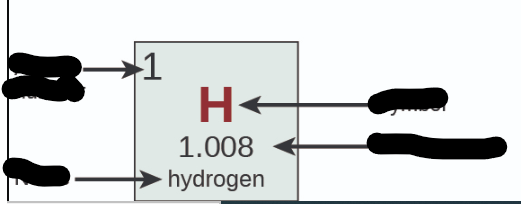
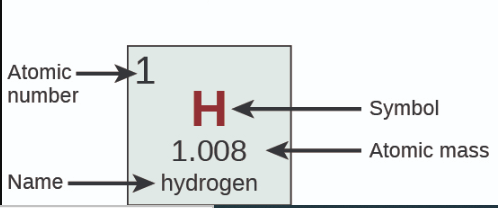
Where is Name?
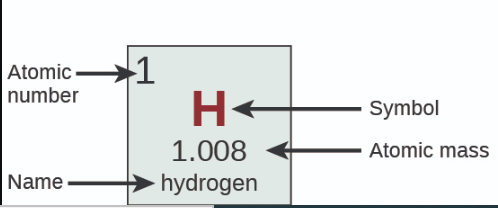
32
New cards
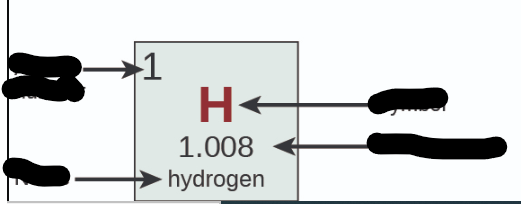
Where is Symbol?
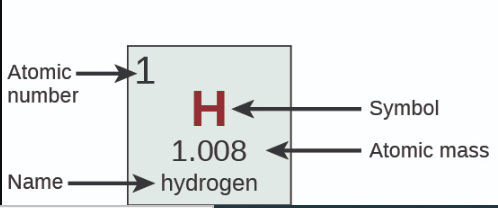
33
New cards
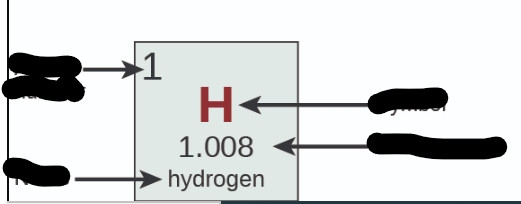
Where is Atomic Mass?