General Chemistry I - Ch.1-4 (Exam 1)
1/85
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
86 Terms
Protons
postively charged particles found in the nucleus
Neutrons
electronically neutral particels found in the nucleus
Electrons
negatively chraged particles distributed around the nuclues;
light, mass is negligible
Atomic Number (Z)
number of protons in the atom (determines the identity of the element) ; Atoms are neutral p+=e-
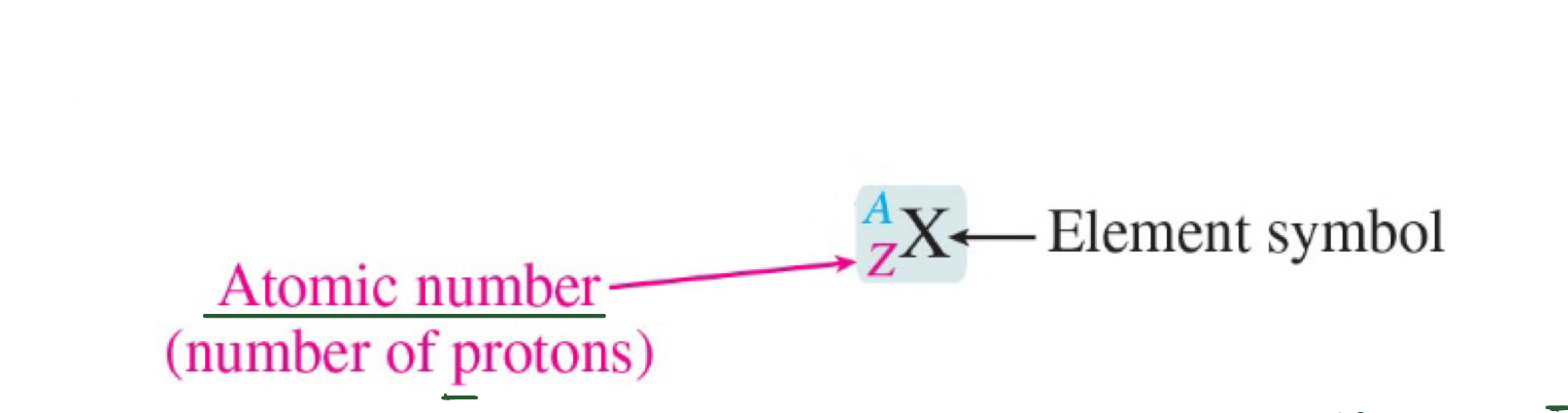
Mass Number (A)
the total number of protons and neutrons
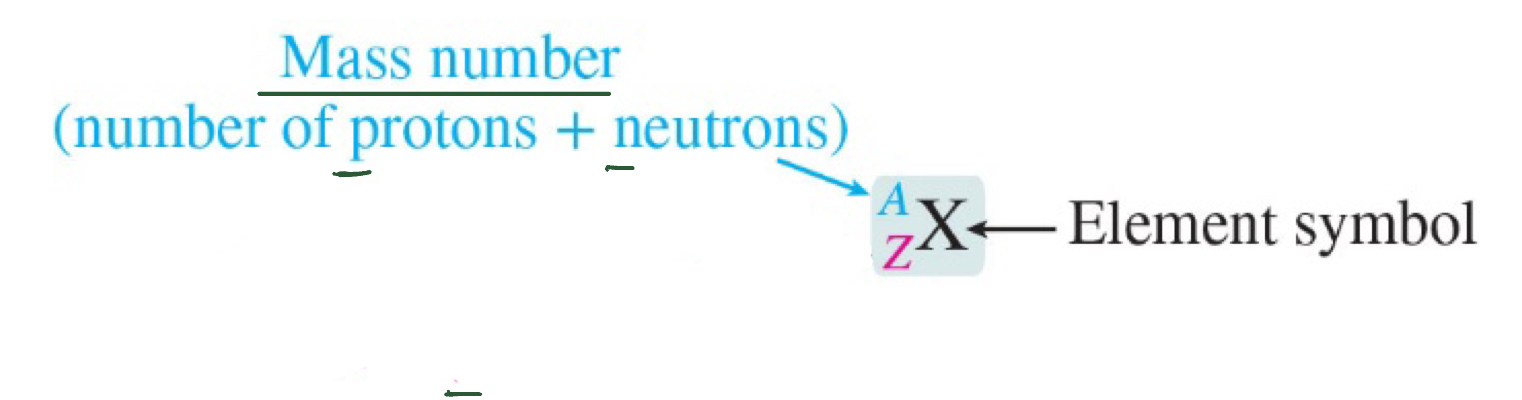
Isotopes
atoms that have the same atomic number (Z), but different mass numbers (A) ; AKA. a different number of neutrons; typically exhibit very similar chemical properties
Atomic Mass
the mass of an atom in atomic mass units (amu). ; the average mass of the isotopes for a given element
Average Atomic Mass
=(isotopic mass)(natural abundance) (for each isotope)
Molar Mass
the mass in grams of one mole of the substance
Grams —> Moles
divide by molar mass
Atoms —> Moles
divide by NA (avagardo’s number)
Moles —> Grams
multiply by molar mass
Moles —> Atoms
multiply by NA (avagardo’s number)
Energy (J)
the capacity to do work or transfer heat ; either kinetic or potential
Kinetic energy (Ek)
the energy of motion
Thermal Energy
the random motion of atoms and molecules ; can be determined by measuring temp. (slow motion, small amt of thermal energy —> dependant on mass and speed)
Cold V. Hot (Thermal Energy)
High temp= larger thermal energy ; Cold temp= small amt. of thermal energy
Absolute Zero (Thermal energy)
Theorhetically- nothing is moving (frozen) at 0 Kelvin @ a fixed postion
Potential Energy
energy possed by an object by virtue of it’s postion: two types: chemcial & electrostatic energy
Chemical Energy
energy stored within the structural units of chemical substances (energy between bonds)
Electrostatic Energy
the potential energy that results from the interaction of charged particles (ex. pos and neg charge) —> attraction force!
Law of Conservation of Energy
energy can neither be created nor destroyed; kinetic and potential energy are interconvertible BUT total energy of universe is a fixed value
Light (radiant energy)
produced by oscillating motion of electric charge; Electromagnetic (EM) radiation; light propagates through space
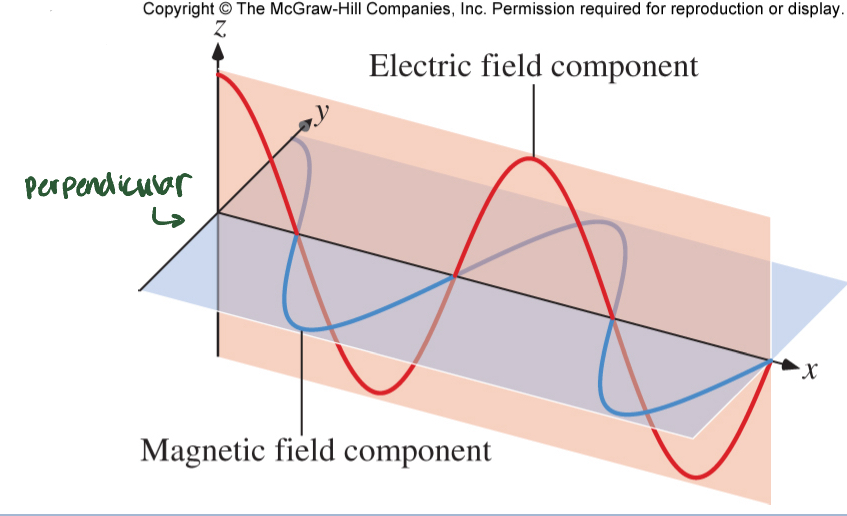
Wavelength
(λ) ; length of a cycle (nm)
Frequency
v ; the numebr of cycles per sec (HZ)
Amplitude
the vertical distance from the midline of a wae to the top of the peak or the bottom of the trough
Energy & Frequency
Direct Relationship (high frequency = high energy = short λ)
Energy & Wavelegth
Inverse Relationship (long λ = low energy = low frequency)
kilo (k)
103
deci (d)
10-1
centi ( c)
10-2
milli (m)
10-3
micro (µ)
10-6
nano (n)
10-9
pico (p)
10-12
Solve for Wavelength
λ = c/v
EM Spectrum: Shortest λ? longest v?
CONTINUOUS: Gamma rays, X-rays, Ultraviolet, Visible (VBGYOR), Infared, Microwaves, Radiowaves
quanta
photons (in small packages/ budles); energy is quantized rather than continuous
Energy of a single quantum of energy is
E=hv
photoelectric effect
electrons are ejected from the surface of metal exposed to light of a min. v, called the threshold frequency. Number of e- ejected is propotional to the intensity of the light
if Ephoton = “binding energy” of e- based on v
e- ARE ejected
if Ephoton > “binding energy” of e- based on v
e- ARE ejected & carry kinetic energy
if Ephoton < “binding energy” of e- based on v
e- are NOT ejected
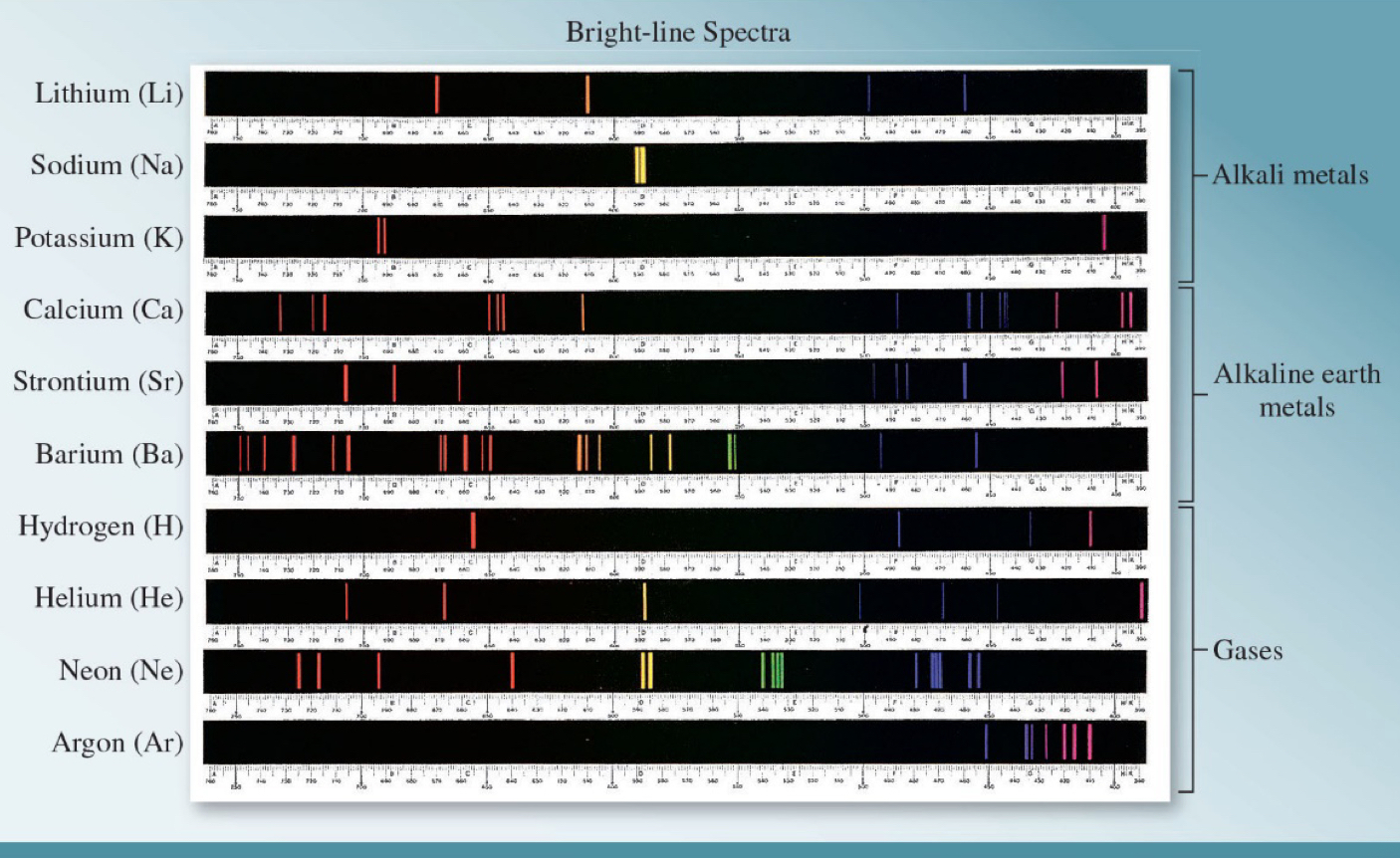
Gas Discharge Tube
electric discharge excites H atoms, transfers energy to e-, as e- relax, energy is emitted as light; NOT CONTINUOUS
Line spectrum = Atomic spectrum
specific to an element
Excitation
e- moves to a higher energy level
Relaxation
e- moves to a lower energy level
n=1
ground states, lowest energy, closest to the nucelus
Bohr’s Theroy of the Hydrogen Atom
electrons move around the nucleus in fixed orbits
when n > 1
excited state (less stable; further from nucleus), higher energy of the orbit
Energy difference between orbits formula
ΔE = -b ((1/n2f) - (1/n2i))
e- moves to a higher n orbit
less stable; e- absorbs energy
e- moves to a lower n orbit
more stable, e- releases energy as photons (light)
nf > ni
ΔE > 0, absorb energy
nf < ni
ΔE < 0, release energy
Heisenburg Uncertainty Principle
it is impossible to know both the momentum and postion of a partical with certainty —> Bohr’s model fail; e- cannot orbit the nucleus in a well-defined orbit
Schrodinger Equation and Quantum Mechanical Despcription of the H atom
gives shape and energy; when e- changes, e- changes to diff wave pattern, “atomic orbital”= e- position in the atom
e- density around nucleus
darker region, higher density, higher probablity of finding e-; each orbital has unique energy and e- density distribution

principal quantum number n
indicates orbital size; larger n, larger orbital; refers to the shell
angular moementum quantum number l
indicates orbital shape; l cannot equal n; s=0, p=1, d=2, f=3
magenetic quantum number ml
indicates orbital orientation’ divides subshell into individual orbitals, intergers from -l to +l
Electron spin quantum number ms
indicates direction of e- spin, -1/2 tor +1/2
Aufbau Principle
build up e- config. from lower energy orbitals
Pauli exclusion principle
maximus of 2 e- allowed in one orbital
Hund’s rule
Unpaired e- if an empty orbital is available
core electrons
inner electrons
valence electrons
outermost electrons; involved in bond formation; determine chemical properties; highest n indicates the valence e-
Group 1A
Alkali Metals
Group 2A
Alkaline Earth Metals
Group 6A
Chalcogens
Group 7A
Halogens
Group 8A
Noble Gases
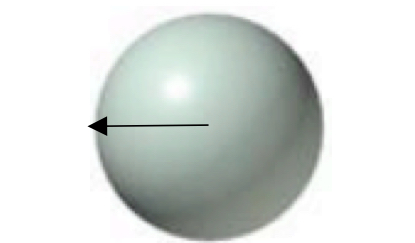
Atomic radius
distance between nuclues of an atom and it’s valence shell
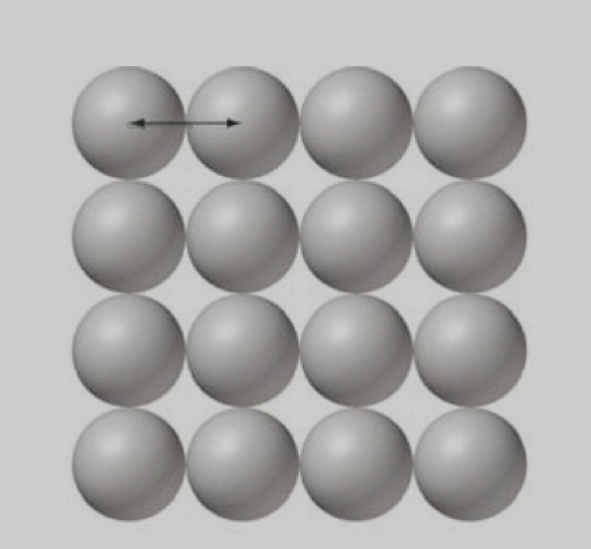
Metallic radius
half the distance between nuclei of two adjacent, identical metal atoms
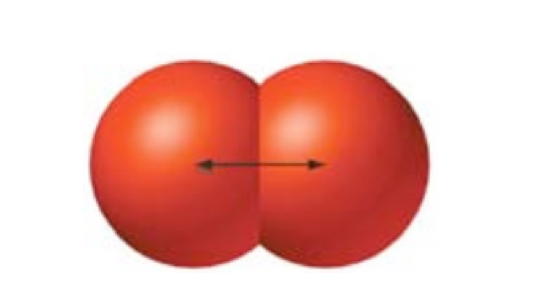
Covalent radius
half the distance adjacent, identical nuclei in a molecule
ion
number of protons and electrons is no longer equal; atom is no longer neutral
ionization energy
energy required to remove an electron from an atom in the gas phase'; removing an e- results in a more pos. ion
cation
postive charge
anion
negative charge
adding/removing successive e-
becomes more difficult & takes more energy
Electron Affinity
Energy released when an electorn is added
x1,000,000
Mega (M)
x1000
kilo (k)
÷10
deci (d)
÷100
centi ( c)
÷1000
milli (m)