B cells
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
how B cells work
identify pathogen
uses many receptors to do this
decision needs to be made
attack planned from spleen and lymph nodes
coordinated approach
cells communicate with eachother and need B cells to launch attack
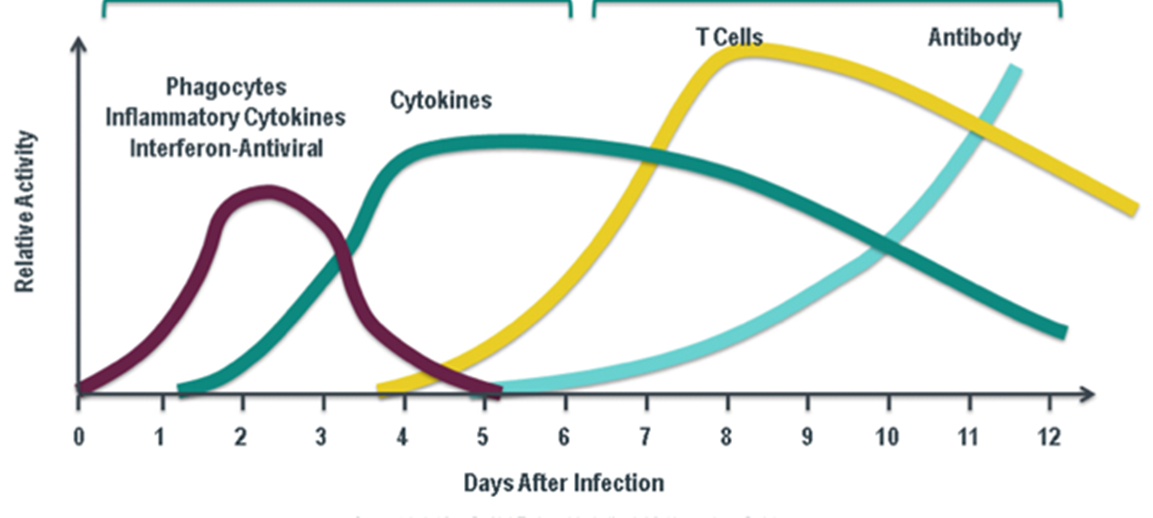
difference between B and T cells
B CELLS
humoral immunity
arise in bone marrow
B cell receptor
produce antibodies for long term - only cell that does this
present antigen - have MHC class 1 and 2
release chemokines
antibodies released into the blood (humoral)
when activated can either become memory cells or plasma cells
T CELLS
cellular immunity
arise in the thymus
t cells recognise niaive t cell biund antigen via t cell receptors
long term memory
dont produce antibody
why do we need B cells
HYPER IgM
in hyper IgM syndrome patients will only produce IgM
patients have many infections and life expectancy is under 30
LUPUS
rifuximab is a treatment for lupus which decreases B cells (as pathological antibodies drive lupus)
people with lupus cant get vaccinated
B cells going wrong is the main cause
people with lupus cant make antibodies as theyre medicated to reduce B cells
therefore, b cells are essential for long term memory
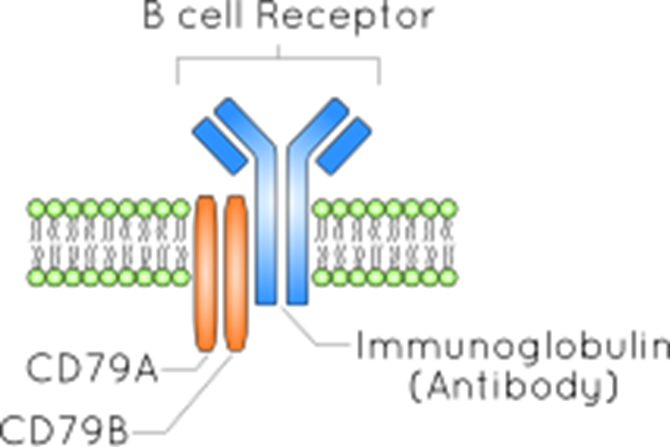
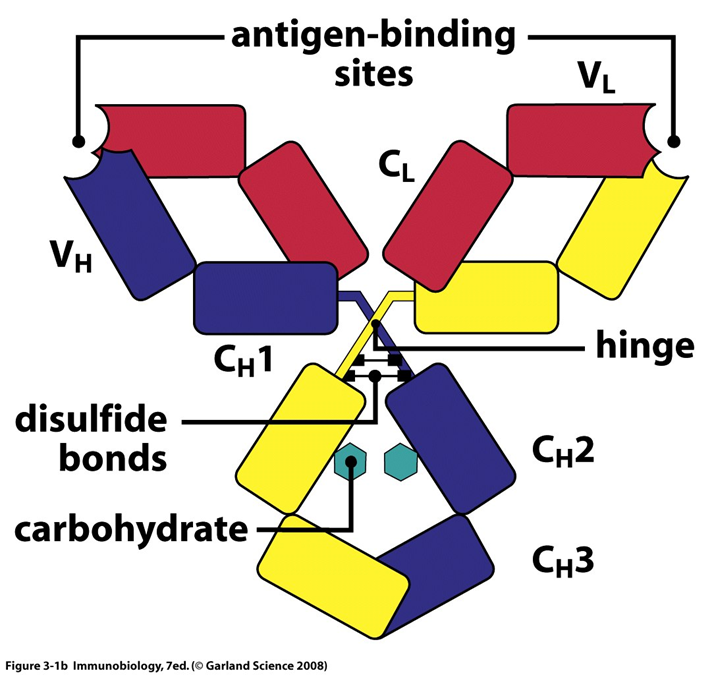
B cell receptor
is a membrane bound antibody
b cells dont bind MHC —> can bind soluble antigen
can present antibody on MHC
antibodies on the membrane has same structure as soluble antibody
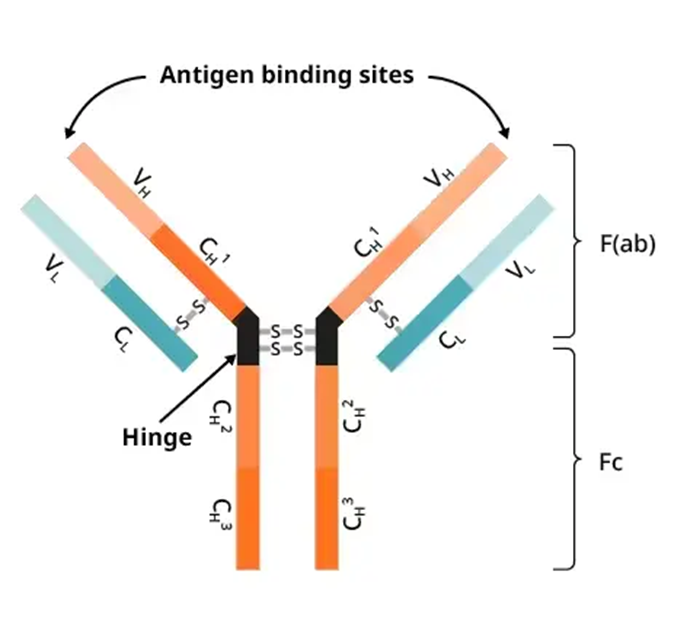
STRUCTURE
2 light chains, 2 heavy (25 and 50kDa)
made up of the antibody the cell is making and CD79
CD79 chains move internal ITAM moelcules which signal to the cell to dictate function
variable and contant region
antigen binding region within variable region
the effector function activity is found within the contant region
the isotype is determines by the carboxy terminal of the heavy chain
ITAMs
immune receptor tyrosine based activation motif
present in b cells ad t cells
2 tyrosine resides separated by 9-12AAs
theyre able to bind with high affinity to SH2 domains of protein tyrosine kinases
when phosphorylated by these kinases they can be bound by further mediators of the signalling cascade
activation of ITAMs is the first signal that the B cell has seen a cognate antigen
a phosphorylation is a quick, good signal for these cells
classes of antibody
5 classes determined by the constant region of the heavy chain: IgM, IgD, IgG, IgA, IgE
each antibody has a different role, migration capacity and is expressed at different times
B cells are able to change what tyope of antibody is expressed
when they begin to mature, they express IgM and later co express IgD
IgD is membrane bound but all other antibodies are secreted
IgA
common at mucosal sites
good in intestine and good defence in the gut
lots of IgA in bodily fluids
IgG
75% of blood based AB
4 subclasses
monomeric —> can diffuse across tissues/across the placenta
antibody mediated cellular cytotoxicity
IgE
least common
main role is binding to mast cells and cross linking Fc receptors
IgE will carry our response to large pathogens such as tape worms where they must be killed by granules
also releases huge amount of histamine, critical for allergic response
IgD
small amounts secreted, usually bound to B cell surface
signals to mature and activate B cells
IgM
pentameric
1st antibody made
good at complement maturation
high avidity
maturation of B cells
early B cells in the bone marrow
BCR locus is arranged as in T cells - heavy chain first then light chain
IgM is expressed on the surface
negative selection and receptor editing occurs befire the cells move to the periphery
IgD expressed on the surface
maturation begins in an antigen dependent fashion
class switching occurs as different antibody classes are made
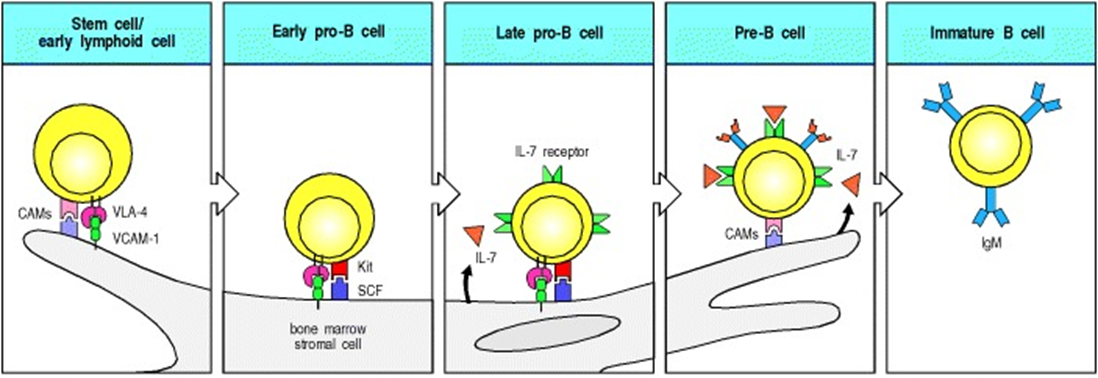
role of the stromal cells
stromal cells (parts of the bone marrow that dont change) in the bone marrow are essential for the survival of B cell progenitors as they move from being heamopoetic stem cells and differntiate into b cells
first, VCAM1 on the stromal cells binds VLA4 on the B cell
this tethers the B cell to the stromal cell so signals can be transferred
the second stromal cell factor from the stromal cells binds cKit on the B cell. This triggers growth and differentiation of the cells
finally, IL7 from the stromal cells binds the IL7 receptor of the B cell
IL7 is a critical cytokine in the bone marrow and it supports the survival and proliferation of B cell progenitors
the B cell is then released into the periphery and VLA4 and cKit are downregulated
diversity of B cells
10 billion different antibody molecules in each of us
BCR recombination drives diversity
only occurs in B cells at the 3 loci that make up the antibody molecule
unlike T cells, B cells can change what they respond to
the 3 stages of recombination:
heavy chain recombination at stem cell stage
heavy chain recombination at early B cell stage
light chain recombination (where IgM expressed)
RAG genes are expressed for a little longer than needed so the B cells can change their antibody
sometimes overhanging gaps will lead to junctional diversity
antibody structure
antibodies have 3 complementarity determinoing regions in the variable region
CDR1 and CDR2 fall where V gene segment is located
CDR3 is the most variable and is formed by the heavy chain
this allows them to be hugely diverse —> in 1ml of plasma can estimate 110 variable gene segments, potential of 5×10^13 antobodies
lots of antibodies will have the same heavy chains but different light chains
negative selection
bout 75% of early B cells generated are self reactive
approx 33% are removed by receptor apoptosis
this is because theres a less comprehensive antigen display system in the bone marrow compared to the thymus
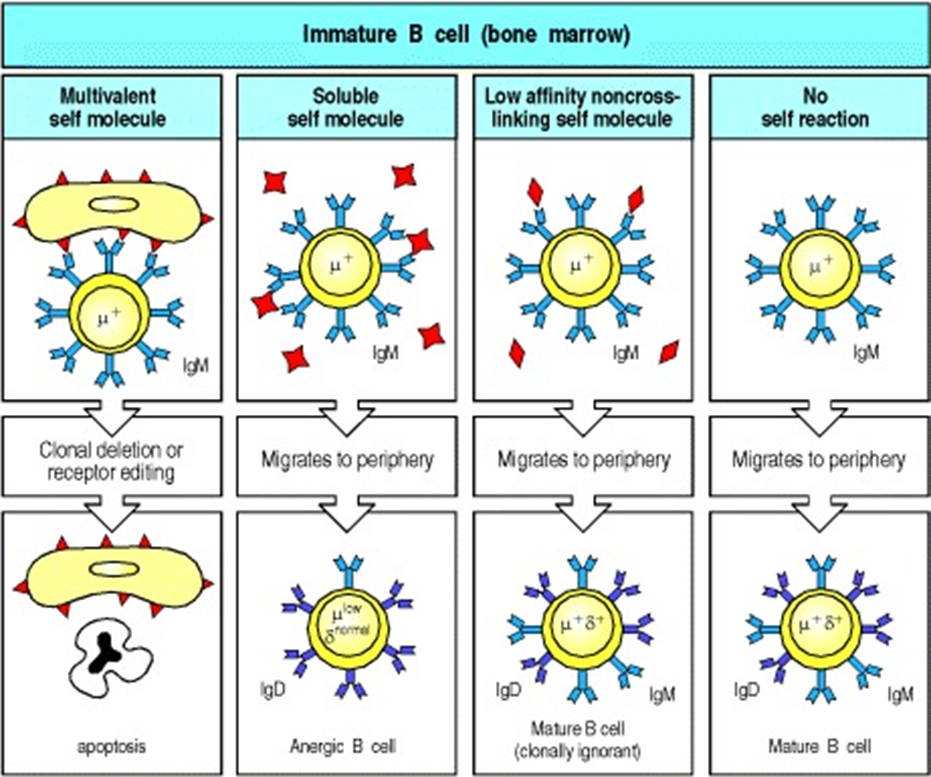
4 routes when B cells are produced in the bone marrow
MULTIVALENT
B cells recognise self antigens
if theyre early enough in development can recombine RAG genes and try again
does not happen in T cells
ANERGIC
if they dont produce a strong signal it becomes angeric in the periphery
could become active again if theres an overwhelming amount of inflammation
cross linking causes a stronger signal
CLONALLY IGNORANT
weak signal from a rare antigen so the B cell wont see the antigen
MATURE B CELL
no self reaction —> normal B cell
receptor editing
if an immature B cell recognises self antigen its surface IgM will cross link which drives a stronger signal through the cytoplasm
most of the time this signal leads to death by apoptosis
but if this happens early enough the right chain rearrangement process can continue and a new BCR can be made
this is receptor editing
anergy and clonal ignorance
like in t cells anergy in B cells is a state where cells continue to survive but are not responsive even if they see their cognate antigen
this is a way to get rid of autoreactive B cells after the early receptor editing stage is passed
anergy is an active process that requires signalling to maintain
anerguc cells have a shorter lifespan
they can be bought out of anergy in very severe infection
we know that they are mature because they express IgM and IgD
short ived and long lived plasma cells
SHORT LIVED
produce lots of antibodies before apoptosis
LONG LIVED
become long lived plasma cells which can produce either IgA, IgG or IgE and also can become memory B cells
t cell help
B cells respond to antigen in the thymus in either a t cell dependent way or a T cell independent way
THYMUS DEPENDENT
antigen can activate the t cell but it cannot be activated any further without a t cell which is delivered through CD40
THYMUS INDEPENDENT
everything required to activate the B cell is delivered by the antigen itself
only a few types of antigen can do this
typically polysaccharides with repeating sequences of sugars so they have multiple repeats of the same antigen
sugar epitopes bind surface IgM and IgD which cluster and a strong activation signal is generated
B cells release IgM and small amounts of IgD
only happens to mature B cells in circulation
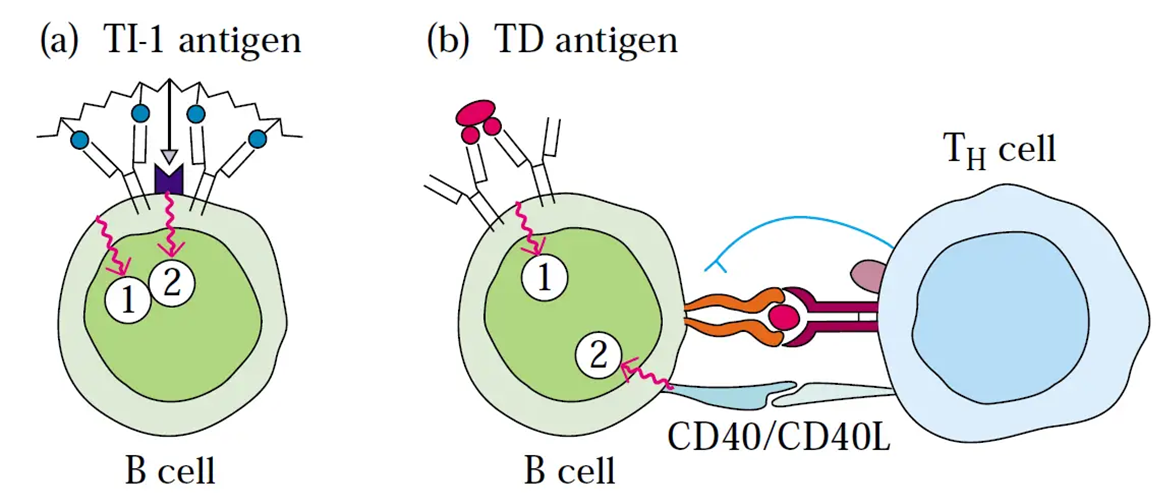
t cell help
requires t cells which recognise the same antigen as B cells (linked recognition)
takes a long time as it acts to conserve energy unless neccessary
t cell delivers signals to B cells which provides the full activation (the main one is CD40 ligand on the t cell which activated Cd40 on B cell)
CD40 a key cytokkine and also triggers B cell proliferation
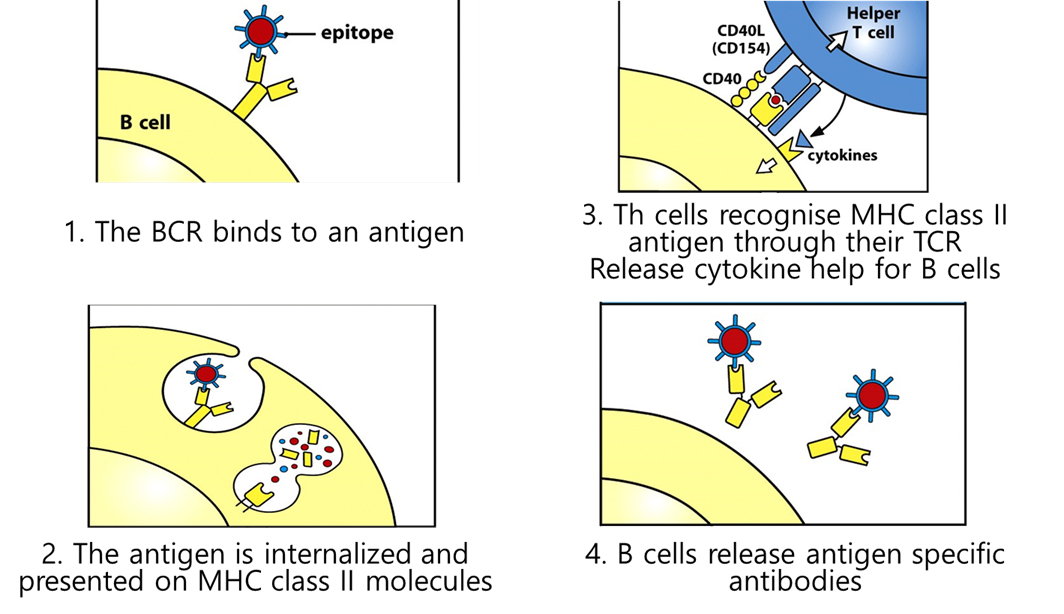
MECHANISM
BCR binds epitope to antigen
it then internalises this and digests it —> proteosome —> ER —> MHC2
the B cell presents the antigen specific atibodies
after teh B cells have turned into plasma cells they downregulate anything they dont need (MHC2)
B cell proliferation in germinal centres
IL5 and Il6 very important for proliferation of plasma cells in germinal centres
t cells have seen an antigen and move into the lymph node via blood vessel of HEV
they remain in the t cell zone, if they see their B cell then they get their signals
the B cells then move to the germinal centre
at first the germinal centres are very small but they grow as b cells proliferate (germinal centres are not present in lymph nodes with no signals)
the germinal centres formed 7-10 days after infection and around the germinal centres is the mantle zone which is made up of resting B cells from previous infections which gradually get displaced by active B cells
in the germinal centres B cells undergo 3 processes:
somatic hypomutation
affinity maturation
class switching
somatic hypomutation
mutation that happens in body cells and is not passed on
undergoes mutation 1 billion times more frequently than other cells —> random
mutations occur in segments that encode the complementarity determining region
particularly CDR3 which binds the epitope of the antigen
some new CDRs will bind antigen better than the orginal antibody
B cells can develop receptors with increased ability to recognise antigen (affinity maturation)
happens because point mutations are introduced into the V region of the light and heavy chin
cytidine deaminase deaminates cytosine to uracil and these uracil molecules will be removed.
this introduces nicks in the backbone which are repaird by an error prone DNA pol
it requires a single strand of DNA and often targets genes that are being actively transcribed E.g: ig genes
affinity maturation
as antibody V regions are mutated, B cells are selected based off the ability to bind antigen
as somatic hypomutation occurs, antibodies become more specific for antigen
vaccination
if we vaccinate aginst an antigen when cells see it agin they can repsond much faster
as the humoral response progresses antibodies with higher affinity are produced
higher frequency, lower number of B cells
the type of antibody they release is changed and they produce the required antibody not IgM
class switching
class switching occurs through DNA recombination
changes to IgG, IgA, IgE during the immune response after t cell help
only occurs in t cell dependent B cells
allows different C region to be used in an antibody that has a specific antigen binding region
the constant region doesnt change unless the class is changed
in this case variable region stays the same as we still want to respond to the same antigen but we want to change the type of AB produced
DNA arrangements where the transcript for IgM is excised and forms a circle which is lost during mitosis
generation of diversity
IN THE BONE MARROW
antigen dependent
pairing of heavy and light chain
recombination
variability on the joins of the recombined gene segments
P & N region nucleotide addition
IN THE PERIPHERY
antigen dependent
somatic hypomutation
class switching and affinity maturation
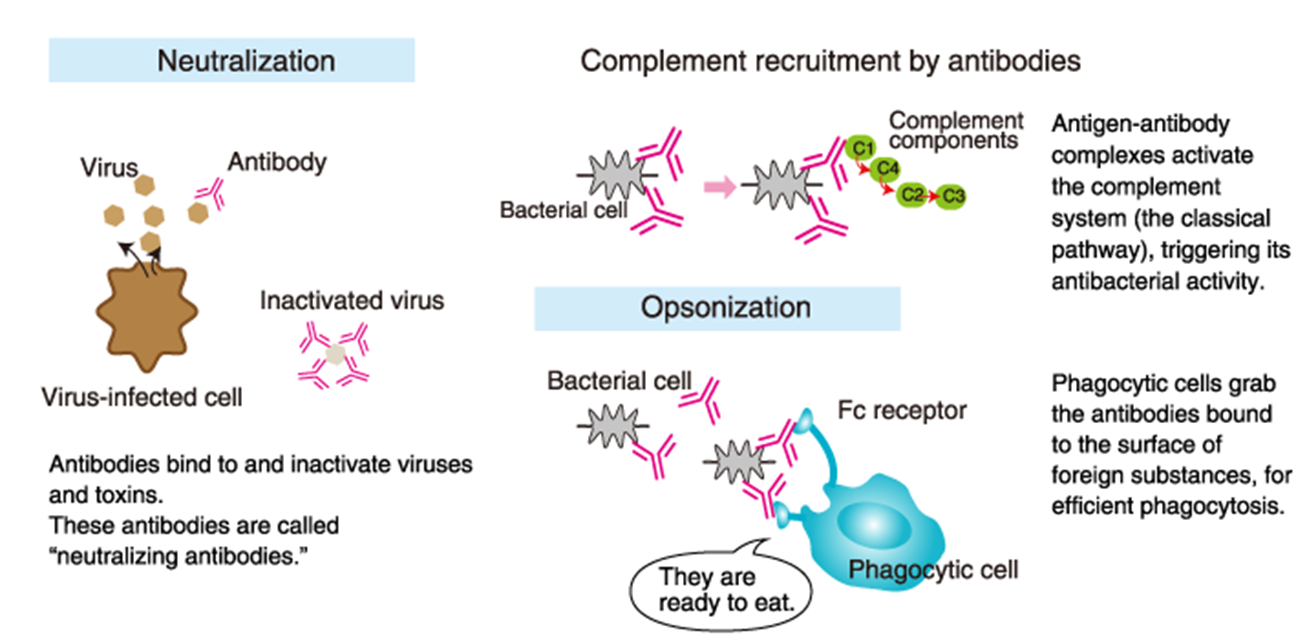
role of antibodies
NEUTRALISATION
bind to soluble antigen and prvent it from working
swallowed up by phagocytic cells
COMPLEMENT
antibodies can bind and activate complement
causing complenet to form a membrane attack complex
OPSONISATION
bind bacterial cells
cause phagocytic cells to take up this complex
has Fc receptors that bind the constant region
Fc receptors
bind the Fc of Ig molecules
these receptors are specifc for diff antibody subclasses and isotypes
formed on many immune cells - Nk, mast cells, neutrophils, eosinophils
some of them phagocytose whatever Fc receptors triggered
other cells like Nk cells and mast cells release granules when Fc receptors are active
very specific to the subclass of antibody
some Fc receptors have an inhibitory negative effect on cells (ITIMs instead of ITAMs)
cytokine production
B cells much slower in cytokine production and its less of a critical function
niaive b cells dont make cytokines
produce chemokines to induce the migration of t cells as well as assist with t cell help
they can also produce cytokines which regulate the response of the immune cells around them
E.g: IL2 boosts T cell proliferation, IL2 is made by B cells
further maturation of B cells
surviving B cells within gthe germinal centres differentiate into plasma cells or memory cells
comtrolled by BLIMP 1 which switches off affinity maturation and proliferation
memory cells are long lived and have undergone affinity maturation
they have a BCR which is highly specific for an antigen but doesnt secrete antibody
plasma cells
secretes 2000 antibodies a second
downregulate MHC 2
dont class switch and undergo no further maturation
short lived
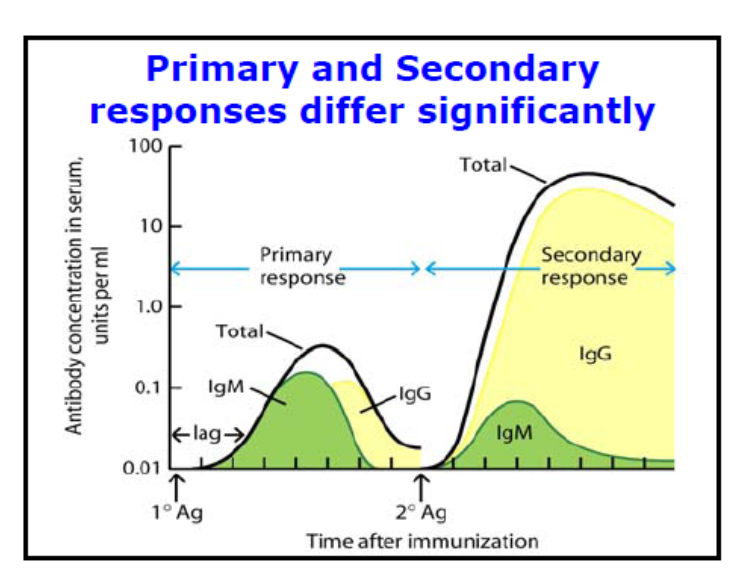
memory B cells
antigen specific memory cells respond to antigen rapidly and proliferate much faster than niaive b cells
the antibodies they make are higher affinity and also continues to increase throughout the secondary response
IgG is produced more quickly in primary challenges
less IgM is produced as the cells have already switched classes
dysregulated B cell response
most ommon is systemic lupus erythmatosus
in healthy people B cells which make antibody to self proteins are deleted but in SLF this goes wrong and B cells survive which means antibodies to nuclear components are made
patients have more B cells and more memory cells which have a lower activation threshold
the antibodies to DNA/RNA form immune complexes which miust be disposed of, most commonly in the kindey which causes tissue damage