Chemlab Practicals (Experiment 1-4)
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms

detection of carbon
-heated dry egg albumin
=char/charring (presence of carbon)
=moisture (presence of hydrogen)

Detection of Nitrogen
-heated urea + soda lime
=ammonia (NH3)
(urea + CaHNaO2——> NH3)

Detection of Sulfur
-dry egg albumin + lead acetate + dil. sodium hydroxide
=lead sulfide (PbS)
=black precipitate/solution
(Pb(Ch3COO)2 + Na2S —NaOH—> PbS + 2Ch3COONa)

Detection of Phosphorus
dry egg albumin + ammonium molybdate + conc. nitric acid
=ammonium phospho-molybdate (3NH4PO4 12MoO3)
=yellow solution)/precipitate
(3NH4= +12MoO4+ + PO43- + 24H+ —→ 3NH4PO4 12MoO3 + 12H2O)

Detection of Halogens (Beilstein Test)
-heated Cu wire
=green flame

Sublimation
-Benzoic acid + Sodium Chloride
=crystals (at the bottom of the Erlenmeyer Flask)
(C6H5COOH + NaCl2 —> pure crystals)

Recrystallization
-impure acetanilide + congo red + animal charcoal
=crystals
(bigger crystals are more pure)
(C6H5NHCOCH3 + congo red + animal charcoal —> pure crystals)

Distillation
-Ethanol (C2H5OH) + red dye
=pure ethanol (C2H5OH) (distillate)
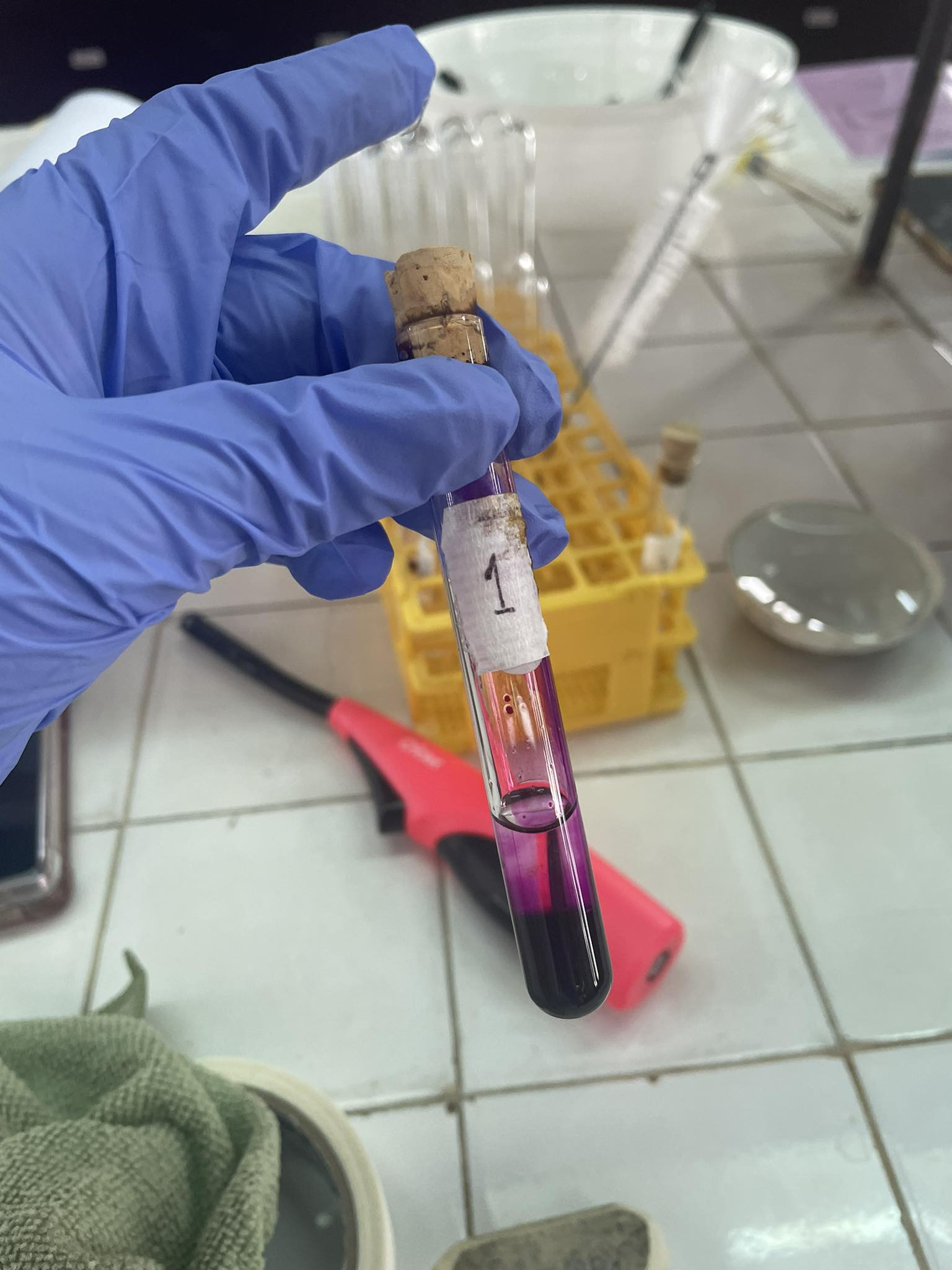
Baeyer’s Test for Unsaturation (alkane: Hexane)
-Potassium Permanganate + Hexane
=Purple Solution (No Reaction)
(C6H14 + KMnO4—> NR)

2H
Bromine Water Test (Alkane: Hexane)
-Bromine Solution + Hexane —-UV light—→
=Bromohexane (C6H13Br)
=Very pale-clear solution
(Br2 + C6H14 = C6H13Br + HBr)
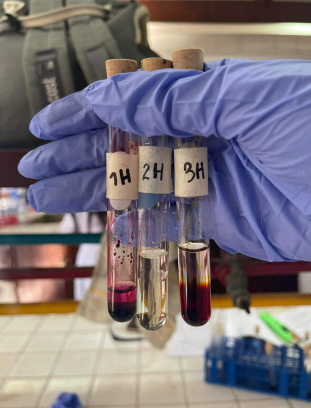
3H
Iodine Test (Alkane: Hexane)
-Alc. Iodine + Hexane
=unmixed reddish-yellow solution (No reaction)

Ignition Test (combustion)
-Hexane + Oxygen
= Carbone Dioxide and Water
=orange luminous flame
(2C6H14 + 19O2 —> 12CO2 + 14H20)
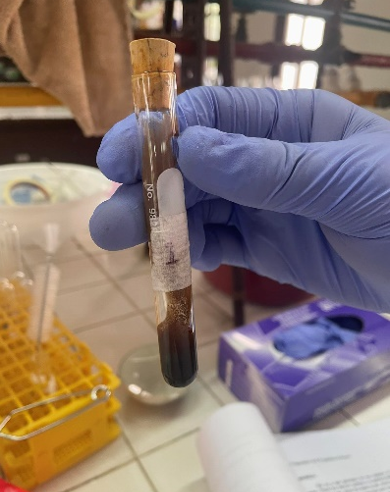
Baeyer’s Test for Unsaturation (alkyne: Acetylene)
-Potassium Permanganate + Acetylene
=Manganese Dioxide (MnO2)
=Brown Precipitate
(2C2H2 + 10KMno4 + 2H2O —→ 10MnO2 + 10KOH + 6 Co2)
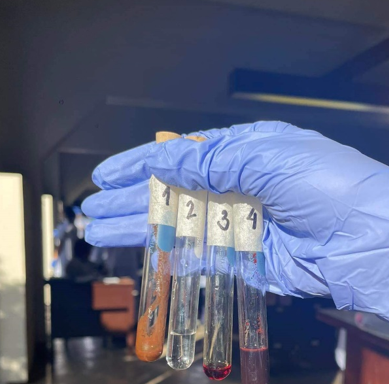
2
Bromine Water Test (Alkyne: Acetylene)
-Bromine Solution + Acetylene —-UV light—→
=1,2-Dibromoethylene (C2H2Br2)
=very pale yellow solution
(Br2 + C2H2 ——> C2H2Br2)
Iodine Test (Alkyne: Acetylene)
-Alcoholic Iodine+ Acetylene —-UV light—→
=1,2-Diiodoethylene (C2H2I2)
=orange-yellow solution
(I2 + C2H2 ——> C2H2I2)

Formation of Cuprous Acetylide
- Copper Chloride + Ammoniacal Solution + Acetylene
=Cuprous Acetylide (Cu2C2)
= chocolate brown precipitate)
(CuCl2 + 2NH4OH + C2H2 —→ Cu2C2 + 2NH4Cl + 2H2O)

Preparation of Anhydrous Alcohol
-95% Ethanol + calcium carbide
=100% Anhydrous Ethanol
(C2H5OH + CaC2 à C2H5OH + Ca(OH)2 + C2H2)

Test for Water in Alcohols
ETHYL ALCOHOL
-Copper Sulfate + Ethanol
= Blue Copper Sulfate
(CuSO4 + C2H5OH —>Blue CuSO4)
ANHYDROUS ETHYL ALCOHOL
-Copper Sulfate + Anhydrous Ethanol
= White Copper Sulfate
(CuSO4 + C2H5OH —>White CuSO4)
CLEAR SOLUTION (ESTERS)
Esterification
ETHYL ALCOHOL
-Ethanol + Acetic Acid + Conc. Sulfuric acid
= Ethyl Acetate (rubbery odor)
(C2H5OH + CH3COOH –Conc. H2SO4—> CH3COOC2H5 + H2O)
(fully oxidized)
METHYL ALCOHOL
-Methanol + Salicylic Acid + Conc. Sulfuric acid
= Methyl Salicylate (minty odor)
(CH3OH + C6H4OHCOOH –Conc. H2SO4—> C6H4OHCOOCH3 + H2O)

Oxidation
ETHYL ALCOHOL
-Ethanol + Potassium Dichromate + Conc. Sulfuric acid
= Acetic Acid (Green)
(C2H5OH + K2Cr2O7 + Conc. H2SO4 —> CH3COOH)
ISOPROPYL ALCOHOL
-Isopropanol + Potassium Dichromate + Conc. Sulfuric acid
= Acetone (Green)
(CH(CH3)2OH + K2Cr2O7 + Conc. H2SO4 —> CH3COCH3)
TERT BUTYL ALCOHOL
-Tert-butyl Alcohol+ Potassium Dichromate + Conc. Sulfuric acid
= No reaction (orange)

Replacement of the Hydroxyl Group with Halogen: Lucas Test
ETHYL ALCOHOL
-Ethanol + Hydrochloric Acid + Zinc Chloride
= No reaction
(C2H5OH + HCl —ZnCl2—> C2H5Cl+ HOH)
ISOPROPYL ALCOHOL
-Isopropanol + Hydrochloric Acid + Zinc Chloride
= No Reaction
(CH(CH3)2OH + HCl —-ZnCl2—→ CH(CH3)2Cl + HOH)
TERT BUTYL ALCOHOL
-Tert-butyl Alcohol+ + Hydrochloric Acid + Zinc Chloride
= cloudiness/ turbidity
(C(CH3)3OH + HCl —-ZnCl2—→ C(CH3)3Cl + HOH)