Nuclear Energy
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Nuclear Fusion
The combining of two small nuclei to form a larger nucleus, with a loss in mass and release of energy.
Example:
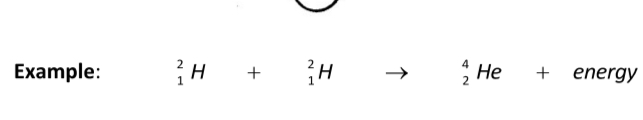
Problems with fusion:
As nuclei have positive charges there is a force of repulsion between any two nuclei. This makes it very difficult for the nuclei to join.
The nuclei must travel at very high speeds in order to join together. This requires very high temperature of 10^8 K. This compares with the temperature within the sun.
Controlling such high temperatures has not yet been achieved and is currently the focus of much research.
Example: The hydrogen bomb is a fusion reaction that has gone out of control. To generate the required heat you have to explode a fission bomb.
Advantage of fusion:
Very little radioactive waste to dispose of.
The required fuel is deuterium (an isotope of Hydrogen), which is easily extracted from water. There would be no fuel shortage.
Nuclear Fission
In nuclear fission, a large nucleus is bombarded with a neutron, it splits into two nuclei of approximately equal size, two or three neutrons are emitted and the resulting loss in mass is released as energy.
Purpose of enriched Uranium:
A vast majority of natural uranium will not fission, which is why fission does not occur by accident on the earth. Enriched uranium is where some of the natural uranium, which isn’t suitable for fission, is removed to increase the probability of fission. This is used in nuclear power stations.
Chain Reaction
Where one fission reaction causes another reaction which causes another reaction and so on.
Nuclear reactor
A chain reaction happens where a fission reaction causes another one fission reaction which in turn causes another one reaction. The chain reaction is controlled.
Nuclear bomb
A fission reaction leads to more than one fission reaction. I.e. it’s deliberately let go out of control.
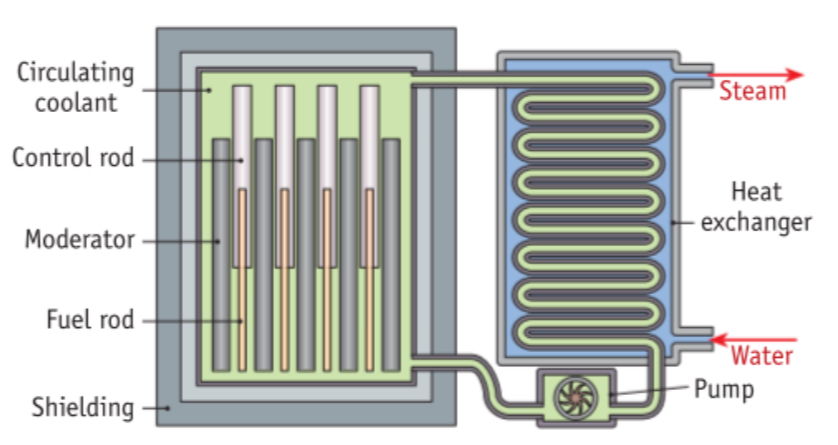
Thermal Nuclear Reactor:
Fuel rods: The fuel is natural uranium or enriched uranium.
Moderator: The moderator is usually graphite or heavy water (D_2O). When a fission reaction happens the emitted neutrons are travelling too fast to cause further fission. The moderator slows down the emitted neutrons to a speed suitable for further fission.
Control rods: Can be made from boron or cadmium. The control rods can absorb the emitted neutrons, i.e. they stop the emitted neutrons. This prevents further fission. This prevents the reaction going out of control. If the control rods are raised then there is a greater rate of fission. If the control rods are lowered then the rate of fission is less. The control rods can regulate the energy output of the reactor.
Coolant: Since the fission fragments have very high kinetic energy the temperature rises within the reactor. Carbon dioxide gas under high pressure is pumped around the system and this carries the heat to the heat exchange unit.
Heat exchange unit: Here the heat carried by the carbon dioxide gas is used to boil water. The steam from the boiling water is used to turn a turbine which generates electricity. Conventional power stations boil water to make steam using fossil fuels.
Shielding: A thick concrete safety shield gives protection from neutrons and gamma rays to the outside world.
Purpose of shielding in thermal nuclear reactor
A thick concrete safety shield gives protection from neutrons and gamma rays to the outside world.
Purpose of the heat exchange unit in thermal nuclear reactor
Here the heat carried by the carbon dioxide gas is used to boil water. The steam from the boiling water is used to turn a turbine which generates electricity.
Purpose of the coolant in thermal nuclear reactor
Since the fission fragments have very high kinetic energy the temperature rises within the reactor. Carbon dioxide gas under high pressure is pumped around the system and this carries the heat to the heat exchange unit.
Purpose of the moderator in thermal nuclear reactor
When a fission reaction happens the emitted neutrons are travelling too fast to cause further fission. The moderator slows down the emitted neutrons to a speed suitable for further fission.
Purpose of the control rods in thermal nuclear reactor
Absorbs emitted neutrons to control the rate of fission in the reactor.
Environmental Impact of thermal nuclear reactors.
To obtain fuel, uranium has to be mined. This exposes miners to radiation from materials like radon. This can cause lung cancer.
An accident at a nuclear reactor could release radioactive chemicals into the atmosphere.
Used nuclear fuel rods have to be carefully stored as they are very radioactive. This is creating problems for future generations. The transportation of these materials is also very hazardous activity.
Uses of radioisotopes (know 3)
Medical imaging: radioactive chemicals with very short half lives are inserted into the body to trace blood flow or to help obtain an image of a particular organ.
Medical therapy: Radiation from certain radioactive chemicals can be used to kill cancerous cells. Great care is taken to reduce damage to healthy cells.
Food irradiation: If food is placed in a sealed container and bombarded with radiation, any harmful bacteria within the food is killed. The food remains fresh for a very long time.
Agriculture: The passage of chemicals(fertilisers) through a plant can be traced making the chemicals radioactive beforehand. This helps in research and development in food production.
Radiocarbon dating: by examining the amount of carbon in old samples, their age can be determined.
Smoke detectors: Smoke detectors contain radioactive material that ionises the gap between two electrodes, allowing a current to flow. If smoke gets between the electrodes, the current is reduced and this triggers the alarm.
Industry: In the manufacturing of paper, the thickness can be checked. The filling of packets of detergent can be checked also. Leaks in underground pipes can be detected.
Health hazards of ionising radiation
The harmful effects of radiation on the human body depends on factors such as:
Nature of the radiation
The part of the body being exposed to the radiation
How active the radioactive material is
The dose the body receives
alpha particles: If the source is outside the body the hazard is slight since a - particles cannot penetrate the skin.
Beta particles: Most of the energy is absorbed by surface tissue. A few mm of aluminium can protect the body.
Gamma rays: These present the greatest external radiation hazard as they can penetrate deep into the body.
Ionising radiation
Any radiation that ionises molecules of a material.
Background Radiation - Radon:
Cosmic Radiation from outer space constantly bombards the earth and we are continually exposed to it.
Granite rock contains minute traces of uranium and this uranium decays to Radon gas which is radioactive. If a house is built over granite, it is possible that radon gas from the granite will slowly build up inside the house. This radon gas will be harmful to the people living in the house. The problem can be solved by proper ventilation to prevent the build up of the gas or by proper insulation to prevent the gas entering the house in the first case.
The level of background radiation has increased since the 1950's when several nuclear bombs were detonated in weapons trials.
The human body contains traces of potassium which is radioactive.
Having an X - ray in hospital or with the dentist is another source of radiation we can be exposed to. However under correct supervision
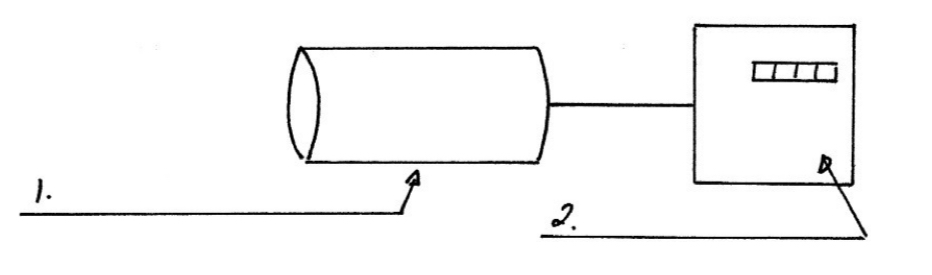
Demonstration experiment to measure level of background radiation:
Connect a Geiger Muller tube to a scaler as illustrated above.
Note and record the count after a time of 1 minute
Repeat this procedure for about twenty minutes and you will observe that the background level will change quite a bit. Work out an average value for the count per minute.
Radiation protection
Precautions include:
Radioactive waste should be correctly disposed of.
Wear protective clothing e.g. gloves, glasses, etc.
Do not eat or drink near radioactive materials as ingested radioactive materials are far more dangerous.
Constantly measure radiation levels near you to determine how much radiation you are being exposed to.