LC Chemistry- Atomic Structure
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
John Dalton Atomic - 1808
-All matter is made up of very small identical particles called atoms.
-All atoms are indivisible. They cannot be broken down into smaller particles.
-Atoms cannot be created or destroyed

William Crookes - 1875
-Showed the existence of cathode rays (beams of electrons) coming from the negative electrode
-Discovered the properties of cathode rays by using the Maltese cross and paddle wheel experiments
-Cathode Rays: travel in straight lines, cause glass to fluoresce, and possess enough energy to move a paddle wheel

J.J. Thomson - 1897
-discovered that cathode rays were negatively charged by using charged metal plates and an electromagnetic field
-he calculated the ratio of mass to charge of an electron e/m= 1.76x10^11 c/kg

George Stoney - 1891
labelled negatively charged particles electrons

Robert Millikan - 1909
-Calculated charge of an electron (e) 1.6x10^-19 C

J.J. Thomson's Plum Pudding Model - 1898
-An atom is like a sphere of positive charge.
-Negative electrons are embedded in the sphere at random.
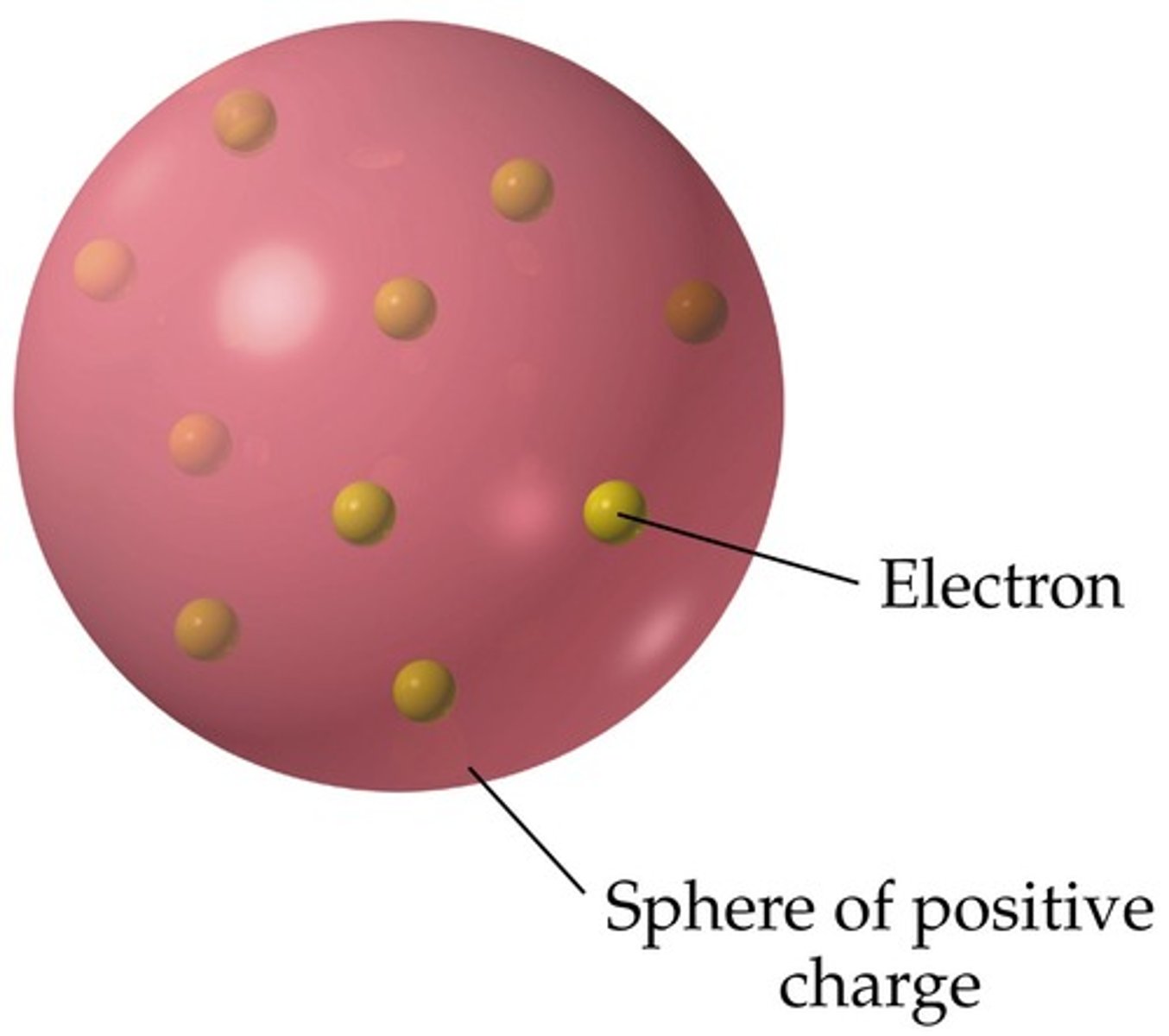
How did Thompson account for the fact that atoms are electrically neutral
plum pudding model of atom
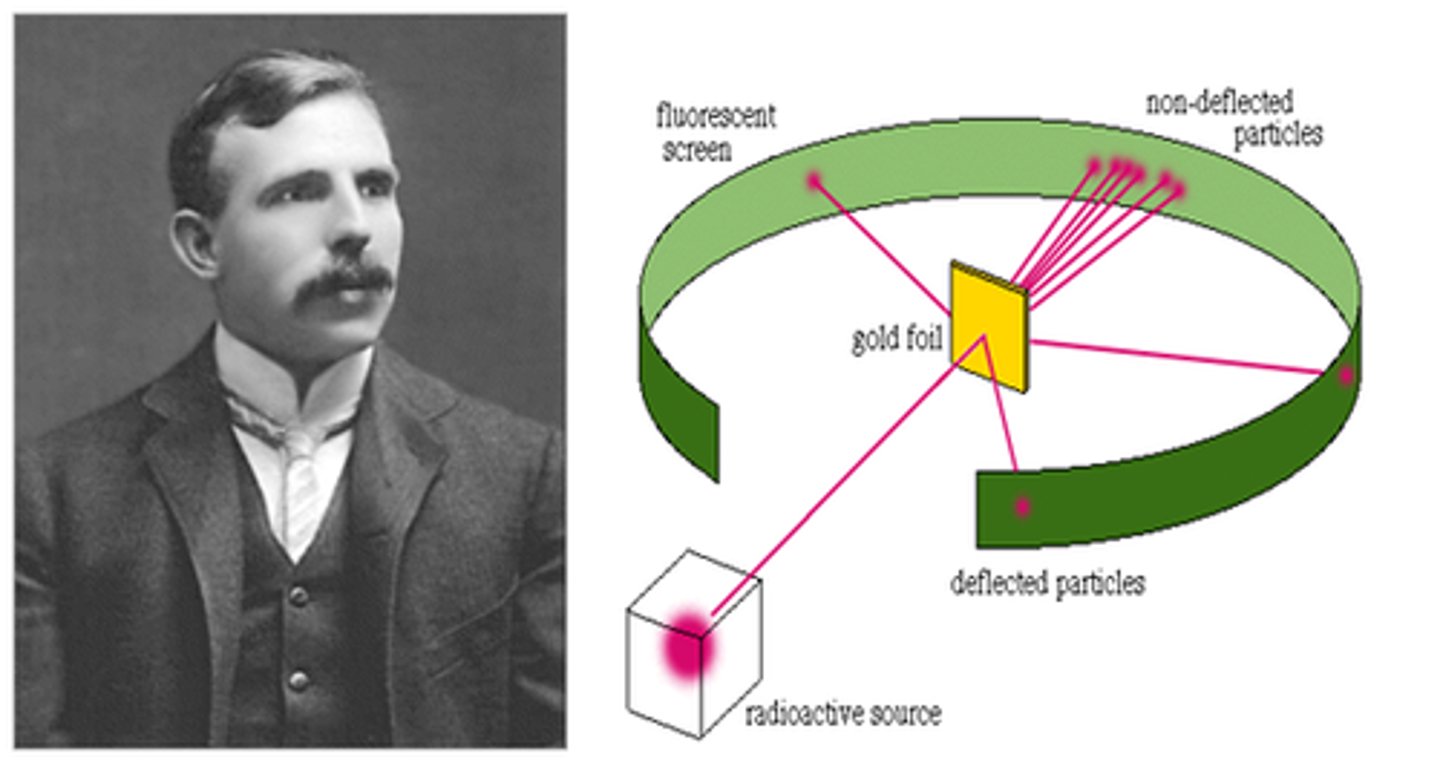
Ernest Rutherford - 1909 1
-discovery of Nucleus
-a thin piece of gold foil was bombarded with alpha particles. -The deflected particles were detected using a phosphorescent screen of zinc sulfide.
Conclusions:
-Most alpha particles passed through the gold foil, proving the atom was mainly empty and the nucleus is small and dense
-the alpha particles are deflected at large angles due to the positive nucleus
-some alpha particles rebounded back as they hit the nucleus
James Chadwick - 1932
-discovery of neutron
-beryllium was bombarded with alpha particles. Neutral particles were being emitted from the nucleus
-Have the same mass as protons
-Called them neutrons
-Neutrons were used to split atoms of uranium and release energy in nuclear reactors and the atomic bomb

Ernest Rutherford 1909 (2)
~Discovery of the proton
~Small positive particles were being released, called protons
Electron- relative mass, relative charge and location
1/1836
-1
orbits nucleus
Proton- relative mass, relative charge and location
1
+1
In nucleus
Neutron- relative mass, relative charge and location
1
0
In nucleus
atomic number
the number of protons in an atom
mass number
The sum of protons and neutrons in the nucleus of an atom
how to find protons
look at atomic number (small number)
How to find the number of neutrons
mass number - atomic number
how to find number of electrons
atomic number - oxidation number
Isotope
Atoms of the same element that have different numbers of neutrons
Relative atomic mass
the average mass of an atom as it occurs in nature, compared with 1/12 of the mass of the carbon 12 isotope
What instrument measures the relative atomic mass if an element
mass spectrometer
what can the mass spectrometer measure
measuring relative abundance
measuring relative atomic mass
calculate the relative atomic mass of a sample of lithium given that the mass spectrometer shows it consists of 7.4% of 6lithium and 92.6% of 7lithium
in 100 atom
92.6x7=648.2 + 7.4x6= 44.4= 692.6
In 1 atom
move 2 decimal places 6.926
Atomic Radius
half the distance between the nuclei centres
atomic orbital
region of space where there is a high probability of finding an electron
why is it difficult to specify the absolute boundary of an atom?
orbitals do not have defined volumes
cathode rays
beams of electrons
why is relative atomic mass rarely whole numbers
average of mass numbers of the isotopes of an electron