Crystals- Defects
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
68 Terms
Crystal imperfections
Deviations from the reference state of a perfect crystal
How do line defects/dislocations impact material behavior?
They facilitate plasticity because they act as obstacles for the motion of dislocations
True or false- line defects act as both sinks and sources for dislocations and vacancies
True!
Grain size strengthening
Strengthening through decreasing grain size and increasing the area of grain boundaries (acts as an obstacle to dislocation motion)
Types of point defects
vacancies, interstitials, substitutions
Self-interstitial
A host atom that occupies a site between lattice points (A atoms in an interstitial site of A atoms)
Impurity intersitial
An atom that occupies a site between lattice points (B atoms in an interstitial site of A atoms)
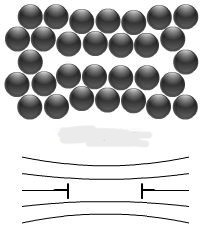
What is this? (+ definition)
Vacancy type dislocation loop- a half-plane of missing atoms in a planar loop
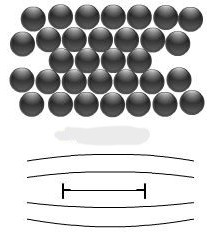
What is this? (+ definition)
Interstitial type dislocation loop- a half-plane of inserted atoms in a planar loop
Vacancies
Vacant unoccupied lattice/atomic sites relative to the perfect crystal reference state
Substitutions
Wrong atom occupancy on a given atomic site relative to the perfect crystal reference state
Interstitials
Atoms in interstitial positions that are unoccupied in the perfect crystal reference state
Types of intrinsic point defects
Vacancies, self-interstitials
Types of extrinsic point defects
Alloying additions, impurities (interstitials, substitutions)
True or false- vacancies do not occur in materials at equilibrium
False- there is an equilibrium concentration of vacancies (x_v)
True or false- the enthalpy of interstitial formation and enthalpy of vacancy formation (h_f) are roughly equal
False- h_f (interstitial) is about 5x h_f (vacancies)
Methods of creating non-equilibrium defect concentrations
Annealing and quenching, irradiation by energetic particles, ion implantation, cold-working
Anti-site defects
Atoms in the wrong sites in chemical compounds (A atom on a B-site), increased chemical disordering
Disordered solid solution
No defined LRO, vacancies in reference to the “average” atom and alloy composition, S close to 0
Ordered solid solution
Defined LRO, vacancies in reference to each atom sublattice, S close to 1
LRO parameter (S)
(r_a - F_a) / (1 - F_a), r_a = fraction of A sites occupied by A atoms
Types of point defects in ionic crystals
Schottky defects and Frenkel defects
Schottky defects
Charge-compensating cation-anion vacancies
Frenkel defects
Charge-compensating ion vacancy paired with an ion interstitial
True or false- defects in ionic crystals always have to be paired with another defect to keep charge neutrality
True!
True or false- comparative probability of defect pairings is determined by how many defects need to take place for charge neutrality
True- fewer defects needed = more probable
0D defects
point defects/site defects
1D defects
dislocations, partial dislocations
2D defects
stacking faults, grain boundaries
3D defects
precipitates, voids
True or false- dislocations are repulsed by each other if on the same glide/slip plane
False- they’re attracted to each other
Impact of annealing/heating on dislocations
Opposite sign dislocations can climb and glide to annihilate, decreasing dislocation density
True or false- the burger’s vector varies with location in a given dislocation
False- it’s constant for the dislocation
Direction of burger’s vector in reference to the tangent vector in dislocations
Parallel/antiparallel in screw dislocations, perpendicular to edge dislocations
Burger’s vector
The displacement vector showing the magnitude and direction of lattice displacement across a dislocation
Line/sense/tangent vector
A unit vector tangent to every point along the dislocation line
True or false- the line vector varies with location in a given dislocation
True- it is always tangent to the dislocation and will vary along curvilinear dislocations
Edge dislocations
Linear dislocations with an inserted/removed half-plane of atoms, causing compressive or tensile strain
Screw dislocations
Linear dislocations with shear displacement
True or false- parallel edge dislocations can experience shear stresses that repel, attract, or exist in metastable equilibrium (with no resultant shear stress)
True
Equation of the glide/slip plane in edge dislocations
b x t
Equation of the glide/slip plane in screw dislocations
undefined- any plane containing b works!
Equation of the glide/slip plane in mixed dislocations
b x t
Unit (perfect) dislocations
Dislocations where b is equal to the lattice translation
Positive edge dislocation
Upside-down T, t into the plane
Negative edge dislocation
T, t out of the plane
Screw component equation
b_s = (b.t)t
Edge component equation
b_e = b - b_s
Conservative motion
Glide motion, there is a displacement of b per dislocation in response to a resolved shear stress parallel to b
Non-conservative motion
Climb motion, requires the creation/destruction of lattice sites and the emission/absorption of vacancies
True or false- climb motion produces equivalent displacement effects to glide motion
True!
True or false- climb loops contain only screw dislocation segments
False- they contain only edge dislocation segments
True or false- dislocations in climb loops are contained in a plane that contains b and t
False- the plane contains t, but not b
Theoretical shear
The simultaneous breaking of bonds across a slip plane
Slip systems
pairings of close-packed planes and close-packed directions on the planes
Unit/perfect dislocations
Dislocations in which b is equal to a lattice translation
Stable cross-slip
Screw dislocations move to neighboring planes
Shockley partial dislocation
b = 1/6 * [112]
Frank partial dislocation
b = 1/3 * [111]
Stair-rod partial dislocation
b = 1/6 * [110]
Dislocation dissociation
Breaking a unit/perfect dislocation into multiple partial dislocations, favorability tested with elastic energy W
Elastic energy W
proportional to b^2
Double cross-slip
Screw dislocations move to a neighboring plane and return to the original plane
Likely slip plane for cF
{111}
Likely slip plane for cP
{011}, {010}
Anti-phase boundaries (APB)
planar faults in ordered compounds that show a shift in stacked planes
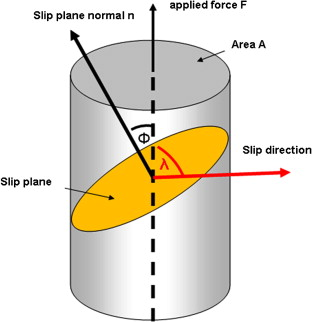
Schmid’s law
shear stress = normal stress * cos(lambda) * cos(phi)
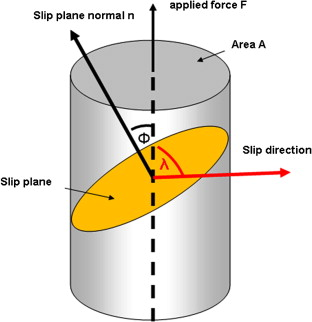
Schmid factor
cos(lambda) * cos(phi), has its maximum value in the primary slip system